Abstract
The aim of this study was to investigate the protective role of Fe in providing tolerance against Cd-stress in root nodules of Vigna radiata, because Cd may be more deleterious in the absence of Fe. Biochemical, histological and proteomic responses to Cd-exposure (50 μM CdCl2) were examined under Fe-sufficient (+Fe/+Cd) or Fe-deficient (−Fe/+Cd) soils by comparing non −Cd exposed control (+Fe/−Cd) plants with additional control of Fe-deficient and non-exposed Cd plants (−Fe/−Cd). Cd-exposure negatively affected on growth and some physiological parameters of host plant and nodules, and also induced oxidative stress with the decline of antioxidative enzyme activities. The negative effects of Cd-exposure in +Fe/+Cd plants were much less than those in −Fe/+Cd and −Fe/−Cd ones. When compared with −Fe/Cd and −Fe/−Cd plants, a marked improvement of bacteriod development and cell division was observed and deformation of cell wall remarkably alleviated in the nodules of (+Fe/Cd) plants. Proteomic study revealed that 20 proteins were differentially expressed by Fe/Cd combined treatment. Eleven proteins of interest were identified and classified as precursor for RNA metabolism, storage of seeds, hypothetical proteins, and unknown proteins. These results indicate that Fe plays a pivotal role in alleviating Cd-stress, as evidence by reduction in oxidative damage and protection of cell wall and bacteriods in nodules.
Similar content being viewed by others
Introduction
Cadmium is non-essential element that negatively affects plant growth and development of plant including root nodules. It is recognized as a significant major pollutant due to its considerable solubility and high toxicity (Pinto et al. 2004). Soil solutions contain Cd concentration ranging from 0.04 to 1 mM. Among several mechanisms of inducing toxicity, Cd can also alter the uptake of minerals by plants through its effect to reduce the availability of minerals from the soil, or through a reduction in the population of soil microbes (Maroco et al. 2002). Cd is well known to induce oxidative stress in plants including legume (Balestrasse et al. 2004). Cadmium can exert toxic effects through its high affinity for sulfuhydral groups in proteins and other biological molecules (Sanita di Toppi and Gabbrielli 1999; Fagioni et al. 2009) and may inhibit metabolic reactions in cell organelles of root-nodules. Cd stress often provokes the generation of reactive oxygen species (ROS) (Qadir et al. 2004). Although some of ROS may function as important signaling molecules that alter gene expression and modulate the activity of specific defense proteins, most ROS can be extremely harmful to organism because ROS can oxidize proteins, pigments, lipids, and nucleic acids which ultimately lead to alteration of cell structure and mutagenesis (Halliwell and Gutteridge 1999). Moreover, Cd-induced oxidative damage is often severe in Fe-defiency because Fe-deficiency provokes a large stimulation of Cd influx in plants (Qureshi et al. 2010) and vice versa affects them at different levels.
Fe is known to be essential for many physiological and biochemical processes including photosynthesis, respiration, DNA synthesis and N2-fixation. The legume-rhizobia symbiosis is particularly sensitive to Fe-deficiency (Tang et al. 1991). Fe-deficiency limits root nodule bacterial survival and multiplication, as well as host plant growth (O’ Hara et al. 1998) because Fe involves in the development of nodules and their function (Ragland and Theil 1993; Krouma et al. 2006). In particular, Fe is required for some key proteins engaged in N2-fixation (nitrogenase, leghemoglobin) and in nitrogen assimilation (glutamate reductase, nitrate reductase) (Vanoni and Curti 2005; Clement et al. 2005). It has been reported that leghemoglobin and ferritin, important lipochito oligosaccharide hemoproteins, which involve in N2-fixation in root nodules of soybean was also affected by Cd stress (Balestrasse et al. 2004).
In recent, Qureshi et al. (2010) reported that Cd proved to be very deleterious to multiprotein complex, except for the PSII sub-complex, the light harvesting II monomer and free proteins under Fe deficiency, and that Fe proved very protective in retaining almost all the complexes. Some histological evidences in root nodules have shown that there is reduction in bacteriods, symbiosomes and reduced number of cell divisions (Balestrasse et al. 2004). Therefore, it may be hypothesized that Fe has a importance for the tolerance against Cd-induced stress especially in leguminous plants such as Vigna radiata (green gram) which ranks high among the pulse crops of India. Although the toxic effects of Cd and interaction between Cd and Fe have been widely documented in photosynthetic tissues (Sárvári et al. 1999; Sanita di Toppi and Gabbrielli 1999; Siedlecka and Krupa 1999; Qureshi et al. 2010; Muneer et al. 2011), relatively less in roots (Roth et al. 2006; Alvarez et al. 2009) but no related informations are in N2-fixing tissues in our knowledge.
In this study, we hypothesized that Fe modulates the Cd-induced oxidative damage together with ultrastructure and proteome changes in nodules of Vigna radiata. To test this hypothesis, the responses of Fe/Cd content in experimental soils, growth parameters, biochemical compounds, antioxidative activity, ultrastructure in root nodules were compared under Fe-sufficient and Fe-deficient conditions. Proteome changes in response to Cd/Fe interaction were also analyzed.
Materials and methods
Plant material and Fe/Cd treatment
Seeds of Vigna radiata L. var. Pusa vishal were obtained from IARI (Indian Agricultural Research Institute, Pusa, New Delhi, India. Surface sterilized seeds were sown in a plastic pot containing 4 kg of experimental soils: (1). iron-deficient soil collected from the Agricultural Soil Science Unit, IARI (Indian Agricultural Research Institute) and (2) normal loamy soil. The seedlings were grown in a phytotron with day/night mean temperature of 25/22 °C under light intensity of 300 m m−2 s−1 with 14 h of photoperiod. Thirty days old plants grown on the Fe-sufficient as well Fe-deficient soils were treated with 50 μM CdCl2 and divided into four experimental treatments as (I) +Fe/−Cd (control) (II) +Fe/+Cd (III) −Fe/+Cd (IV) −Fe/−Cd. Sampling was performed at 24 and 72 h after Cd-exposure.
Estimation of iron content and Cd in experimental soils
Experimental soils (500 mg) from all four treatments was digested in acid mixture (HNO3/HCl, 2/1, v/v), filtered (Whatman no. 42), and then diluted to appropriate volume. The Fe/Cd contents in the solution were estimated in ppm using a flame atomic absorption spectrometer (Video11; Thermo Jarrell Ash Corporation, Franklin, MA, USA).
Growth and physiological parameters
Plants were uprooted carefully by moving a spatula along with pot wall and roots were washed by dipping in a tube containing distilled water followed by blot-drying with very soft lint-free paper. Each plant was separated into root and nodules with the help of sharp scalpel and forceps in moist paper sheets, and then weighed for nodule and root biomasses.
Chlorophyll content was estimated by the method of (Hiscox and Israclstam 1979). Fresh leaves were collected in glass vials to which 10 ml DMSO were added and were kept in an oven at 65 °C for complete leeching of pigments 1 h. Optical density was recorded at 480, 645, 520 and 663 nm. The chlorophyll concentrations in mg fresh samples were calculated using formulae given by Aronon and Israelstam (1979).
The lipid peroxidation level was determined by measuring the concentration of malondialdehyde (Heath and Packer 1968). One gram of fresh tissue was ground in 0.1 % trichloroacetic acid (TCA) and centrifuged at 10,000g for 5 min. The mixture containing 1 ml of supernatant with 0.5 % thiobarbituric acid (TBA) was heated at 99 °C for 30 min, cooled and centrifuged at 5,000 rpm for 5 min. The absorbance of the supernatant was read at 532 nm and corrected for unspecific turbidity after subtraction from the value obtained at 600 nm.
Biochemical analysis
Ascorbate was determined by the method of Law et al. (1983). Five hundred milligram of grounded tissues was homogenized in phosphate buffer (pH 7.4), centrifuged at 10,000g. Resulting supernatant was added with bipyridyl and FeCl3. The absorbance at 525 nm was measured.
Glutathione was estimated by the method of Anderson (1985). Five hundred milligram of fresh tissues was homogenized in 5 % sulphosalicylic acid and centrifuged at 10,000g. Glutathione was estimated by adding dithionitrobenzoic acid (DTNB). The reaction was started by adding glutathione reductase for 30 min at 25 °C. The absorbance at 412 nm was immediately read.
Leghemoglobin was estimated using a flurometric method as described by (La Rue and Child 1979). Three hundred milligram of fresh nodules was homogenized in extraction buffer containing 0.02 % (w/v) potassium (K), ferricyanide and 0.1 % sodium bicarbonate. Homogenate was centrifuged at 10,000g for 10 min. Leghemoglobin in the red supernatant (nodule cytosol) obtained after centrifugation was quantified by using bovine hemoglobin as a standard.
For measurement of ferritin concentration, 300 mg of fresh nodule was homogenized in cold 10 mM KCl HEPES (pH 7.9) solution containing 10 mM KCl and 0.5 mM dithiothriotol. Homogenates were centrifuged at 15,000g for 5 min. and Ferritin was estimated on Gel pro software by using standard horse ferritin and goat anti horse ferritin as primary and secondary antibody (Roskams and Connors 1994).
Enzyme assays
Ascorbate peroxidise (APX) activity was estimated by measuring the decrease in absorbance at 290 nm (extinction coefficient of absorbance 2.8 mM−1 cm−1 for ascorbate) according to Nakano et al. (2006). One hundred milligram of fresh tissue were extracted in 100 mM K-phosphate (pH 7.0), and then centrifuged at 15,000g for 10 min. One unit of enzyme was expressed as the amount necessary to decompose 1 μmole of ascorbate per min.
Superoxide dismutase (SOD) activity was determined by the method of Dhindsa et al. (1981) with minor modifications. Fresh tissues (200 mg) were extracted in phosphate buffer (pH 7.3) and centrifuged at 15,000g. SOD activity in the supernatant was assayed by its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT) by reading the absorbance at 560 nm. One unit of enzyme activity was defined as the amount of enzyme required to inhibit 50 % of the NBT photo reduction in comparison with tubes lacking the plant extract.
Catalase (CAT) activity was determined by the method of (Aebi 1984). About 500 mg of grounded tissues were extracted in phosphate buffer (pH 7.3), centrifuged at 15,000g for 20 min. The decrease in absorbance at 240 nm was recorded as a result of H2O2 degradation (extinction coefficient of 36 mM−1 cm−1). One unit of enzyme determines the amount necessary to decompose 1 μm of H2O2 per min.
Glutathione reductase (GR) activity was determined by the method of Rao (1992). About 500 mg of fresh tissues were extracted in phosphate buffer (pH 7.0), centrifuged at 15,000g for 10 min. The supernatant was immediately assayed for GR activity through glutathione-dependent oxidation of NADPH at 340 nm. One unit of enzyme determines its amount necessary to decompose 1 μmole of NADPH per min.
Electron microscopy
Harvested mature nodules at 72 h of Cd-exposure were was cut into app. 1 mm pieces with a sharp blade and stored in fixing solution containing 1 % formaldehyde, 2.5 % glutaryldehyde and 2 % paraformaldehyde overnight at 4 °C. Nodule samples were washed with 100 mM phosphate buffer (pH 7.4), placed in osmium tetraoxide for 2 h at 4 °C, After washing again with phosphate buffer, dehydration with acetone 30–90 % was followed. Finally samples were dehydrated in dry acetone (saturated with copper sulphate) for 1 h at 4 °C. Samples were cleaned with toluene, twice for 1 h each and placed in resin and toluene (1:3, w/v) overnight in vacuum followed by impregnation again in resin and toluene (2:2 and 3:1) overnight in vacuum before being finally impregnated in pure resin for 6 h at room temperature. Samples were embedded with resin in flat molds. Thin sections of the size of 1–2 μm were cut and stained in with methylene blue for 20–40 s. Grids were made of the size of 60–90 nm and stained with heavy metal solution (uranyle acetate and lead citrate). The stained sections were placed on a transmission electron microscope (Model CMIO, TEM, Phillips). Interior regions with comparable structures were surfed and the pictures were digitized.
Proteomic analysis
Mature nodules harvested at 72 h of Cd-exposure were grounded in liquid nitrogen to a fine powder extracted for protein with 40 mM Tris–HCl extraction (pH 7.5) according to Molloy et al. (1998). Homogenate was centrifuged at 20,000 rpm for 60 min at 4 °C. Resulting supernatant was incubated over night with chilled 12 % TCA-acetone, centrifuged at 15,000 rpm for 15 min. Resulting pellet was washed with chilled acetone containing 0.07 % β-mercaptoethanol and 2 mM EDTA, and then centrifuged at 20,000g for 15 min. Pure white pellet recovered was vacuum dried and solubilized in solubilization cocktail containing 9 M urea, 2 M thiourea, 2 % Triton X 100, 4 % CHAPS, 0.2 % ampholine and 50 mM DTT at room temperature for 60 min. Protein concentration was determined by standard Bradford assay using bovine serum albumin as standard (Bio-Rad, Hercules, CA, USA). Immobilized pH gradient (IPG) strips (ReadyStrip™, Bio-Rad, USA) of 11 cm, non-linear with pH 3–10 were passively rehydrated overnight with 150 μg of protein sample. Isoelectric focusing (IEF) of proteins was performed using the following programme: 50 volts for 60 min, 150 volts for 30 min, 300 volts for 30 min, 500 volts for 120 min, 3,000 volts until a total of 72,000 volt-hours had been achieved. After IEF, strips were equilibrated in buffer containing 7 M urea, 2 % SDS, 375 mM Tris (pH 8.8), and 20 % glycerol plus either 130 mM DTT for reduction or 135 mM IAA for alkylation. Equilibrated IPG strips were loaded onto a 12 % acrylamide gel, and electrophoresed by applying a voltage of 100 volts at 10 °C. Gels were stained with Coomassie Brilliant Blue R-250 and images were acquired on a gel documentation system (Bio-Rad, USA) at 300 dpi and saved as a gray scale. Experimental molecular weight and pI values were calculated from digitized images using molecular weight marker proteins and the predicted non-linear pH gradient provided by Bio-Rad USA. Protein spots were detected and numbered with PD Quest image analysis software (Bio-Rad, USA). The protein expression patterns were determined as up-regulated, down-regulated and unchanged compared to control. Three independent experimental replicates were used for proteomic analysis. The differentially expressed proteins were spot picked, digested with trypsin and identified by LC/MSMS.
Stastical analysis
SAS 9.0 software (SAS Institute Inc, 2002) was used for statistical analysis. The experiment was done as randomized block design. The mean and standard deviation was done for bar diagrams with percent variation. Analysis of variance (ANOVA) was done with all the data to confirm the variability of the data and validity of results.
Results
Fe and Cd content
The content of Fe in all four treated soils were estimated and it was observed that Fe content in −Fe/−Cd was decreased compared to control and was more severely decreased in −Fe/+Cd plants (Fig. 1a) whereas in +Fe/+Cd plants the content of Fe was increased.
The Cd content increased in +Fe/+Cd compared to control which was more severely increased in −Fe/+Cd plants however, in −Fe/−Cd and in control plants the content of Cd was found in trace amounts (Fig. 1b).
Plant growth parameters, chlorophyll and protein
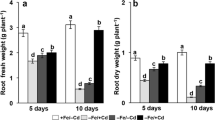
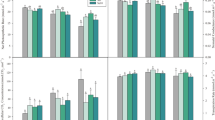
Cd-exposure significantly decreased shoot and root length compared to those of non −Cd-exposed control (+Fe/−Cd) plants. These decreases were more severe in Fe-deficient (−Fe/+Cd) compared to those in Fe-sufficient soils (+Fe/+Cd) and −Fe/−Cd (act as additional control) (Table 1). A significant decline in total chlorophyll content was apparent only in −Fe/Cd plants. A drastic decrease of about 79 and 81.5 % was observed in protein content in −Fe/+Cd (Fig. 2a), whereas the decreases was much less in +Fe/+Cd plants.
Changes in protein content (a), leghemoglobin (b) and ferritin concentration (c) in root nodules of non −Cd-exposed (control) or Cd-exposed under Fe-sufficient (+Fe/Cd) and Fe-deficient (−Fe/Cd) with additional control (−Fe/−Cd) after 24 and 72 h of Cd-exposure. Vertical bars indicate mean ± SE for n = 4
Nodule development and concentration of leghemoglobin and ferritin in nodules
Nodule number significantly decreased only in −Fe/+Cd plants as compared to control (Table 1). Cd-exposure significantly decreased nodule biomass, leghemoglobin and ferritin concentration compared to control and the reduction was more in −Fe/+Cd plants and less in −Fe/−Cd plants. In −Fe/+Cd plants nodule biomass was reduced by 70 and 80 %, respectively, after 24 and 72 h of sampling whereas in +Fe/+Cd plants only 40 and 50 % were reduced (Table 1). Leghemoglobin concentration at 72 h of Cd-exposure was reduced by 19.4 % in +Fe/+Cd, while up to 63.4 % in −Fe/+Cd plants compared control however, less decreased in −Fe/−Cd plants compared to that of control (Fig. 2b). Cd-exposure remarkably decreased ferritin concentration by 81.6 and 92.2 % at 24 and 72 h in −Fe/+Cd plants, while decreased by only by 11.9 and 33.3 % in +Fe/+Cd plants whereas, less affect was observed in −Fe/−Cd as compared to those of control (Fig. 2c).
Lipid peroxidation, ascorbate and glutathione
Lipid peroxidation was significantly increased by Cd-exposure under Fe-deficient condition (−Fe/+Cd), but no significant change occurred under Fe-sufficient (+Fe/+Cd) compared to control (Fig. 3a) however, in −Fe/−Cd plants lipid peroxidation was less affected compared to −Fe/+Cd plants when compared to that of control. Total glutathione concentration were reduced by 28.5 and 22.3 % at 24 and 72 h after Cd-exposure in −Fe/+Cd, whereas they increased by 86 and 49.6 % in +Fe/Cd plants compared to controls and was less affected in −Fe/−Cd plants (Fig. 3b). Similarly, total ascorbate concentration were reduced by 18.8 and 46.5 % at 24 and 72 h after Cd-exposure in −Fe/+Cd and least decreased in −Fe/−Cd plants (Fig. 3c), whereas they increased by 9.4 and 34.1 % in +Fe/+Cd plants compared to controls.
Changes in lipid peroxidation (a), total glutathione (b) and total ascorbate (c) in root nodules of non −Cd exposed (control) or Cd-exposed under Fe-sufficient (+Fe/Cd) and Fe-deficient (−Fe/Cd) with additional control (−Fe/−Cd) after 24 and 72 h of Cd-exposure. Vertical bars indicate mean ± SE for n = 4
Antioxidant enzymes activity
The response of all antioxidative enzymes examined in this study was in exquisite contrast between Fe-sufficient and Fe-deficient condition. Cd-exposure increased APX, SOD, CAT and GR activity under Fe-sufficient (Fig. 4a, b, c, d), while inversely decreased under Fe-deficient condition at all-time courses of measurement when compared with control and in −Fe/−Cd plants the antioxidant enzymes were less decreased compared to control. At 72 h of Cd-exposure, APX, SOD, CAT and GT activity was increased by 10.0, 26.3, 30.2 and 22.7 % respectively under Fe-sufficient soil compared to those of control, but decreased by 64.1, 35.4, 29.8 and 64.3 % respectively under Fe-deficient soil.
Changes in antioxidant enzyme activities; ascorbate peroxidase (APX) (a), superoxide dismutase (SOD) (b), catalase (CAT) (c) and glutathione reductase (GR) in root nodules of non −Cd exposed (control) or Cd-exposed under Fe-sufficient (+Fe/Cd) and Fe-deficient (−Fe/Cd) with additional control (−Fe/−Cd) after 24 and 72 h of Cd-exposure. Vertical bars indicate mean ± SE for n = 4
Ultrastructure of nodules
Electron micrograph revealed that Cd/Fe individual and/or combined impact closely associated with the histological changes of nodules (Fig. 5). In control plants, the bacteriods were well developed and distributed evenly in cytoplasm (Fig. 5a) and cell wall was keeping normal shape in a general form (Fig. 5e). Cd-exposure (+Fe/+Cd) resulted in the reduction of cell division and dislocation of bacteriods in cytoplasm (Fig. 5b) and provoked also deformation of cell wall (Fig. 5f) in the nodules. In the plants under Fe-deficient condition (−Fe/+Cd), number of bacteriods largely decreased and cell division was strictly restricted (Fig. 5c), together with a distorted and ruptured shape of cell wall (Fig. 5g). When compared with −Fe/+Cd plants, a marked increase in bacteriods and cell number was observed (Fig. 5c) and deformation of cell wall remarkably alleviated in the nodules fed with sufficient Fe (+Fe/+Cd) even though distorted parts were still observed (Fig. 5f). In −Fe/−Cd plants the bacteriods were reduced in number (Fig. 5d) and cell wall was also deformed at certain positions (Fig. 5h) because of absence of Fe when compared to control however, was least affected when compared to −Fe/+Cd plants. These provided important clues of the potential role of Fe in mitigating the Cd-induced damage in nodule structure.
a Figures show micrograph developed electron-Microscopy (TEM) technique of electron microscopy (EM). TEM of nodule obtained from control (+Fe/Cd) plants show normal growth with regular features of typical nodule (a) such as normal cell growth with embedded bacteriods in cytoplasm. b depicts (−Fe/Cd) absence of bacteriods, reduced cell divisions, c depicts (+Fe/Cd) reduction in bacteriods, cell divisions eventually ceased up, d depicts (−Fe/−Cd) reduction in bacteriods and act as an additional control. b Figures show micrograph developed electron-Microscopy (TEM) technique of electron microscopy (EM). TEM of nodule obtained from control (+Fe/Cd) plants show normal cell wall formation (e,d). f depicts (−Fe/Cd) Reduction in cell wall formation, g depicts (+Fe/Cd) reduction in cell wall compared to control however, recovered by giving Fe, h depicts (−Fe/−Cd) as additional control and cell wall formation reduced
Proteomic analysis of root nodules
First dimensional electrophoresis were run on IPG strips to focus the proteins from root-nodules harvested after 72 h of Cd-exposure and the focused proteins were separated by the 2nd dimension SDS-PAGE. Figure 6 showed 2D gel profile of differentially expressed proteins in root-nodules obtained from different experimental (treatments) sets, viz. (I) control (II) +Fe/Cd (III) −Fe/Cd (IV) −Fe/−Cd. While comparing the 2D gels of root-nodules it was found protein profile was more affected under −Fe/Cd stress compared to +Fe/+Cd. This implies that Fe seemed to greatly help plants in counteracting the negative effects of Cd treatment by retaining quantity of proteins and quality of root-nodules While comparing with −Fe/+Cd plants the protein profile was well astonished in −Fe/−Cd plants (Fig. 5d). Approximately 150 spots were detected on 2-D gels of nodule proteins among these spots, about 20 spots were found to be differentially expressed (Fig. 6). Furthermore, 11 protein spots out of 20 differentially proteins were positively identified by LC/MSMS as listed in Table 2 with information about matched peptides and ion score, nevertheless it was identified that differentially expressed proteins were predicted to be RNA rich binding and hypothetical proteins. To further examine differentially expressed proteins due to combined effect of Fe/Cd, the identified proteins were divided into four functional categories using Map Man ontology classified by Beven et al. (1998) as shown in Fig. 7, including RNA metabolism, hypothetical proteins, secondary metabolism and proteins yet not assigned with function.
Differentially expressed proteins in root nodules (a = +Fe/−Cd, b = −Fe/+Cd, c = −Fe/Cd, d = −Fe/−Cd) of Vigna radiata in response to Fe deficiency and Cd stress. Protein samples (150 µg) were separated on 2D gel (pI 3–10) and Coomassie stained. Data analysis was performed using PD quest software program. The relative Mr is indicated on left side in kDa. 20 differentially proteins were analyzed by LC/MSMS among which only 11 spots were identified
Functional classification of identified proteins from root-nodules analyzed by LC/MS–MS using Map Man ontology as described by Beven et al. (1998)
Correlation between Fe/Cd and other parameters
Linear correlation among Fe/Cd with related parameters was assessed. The measurements were normalized between the values measured in Fe-deficient or Fe-sufficient plants with exposure and non-exposure to Cd. The Fe concentration was positively correlated to Soluble protein (r = 0.81***), total ascorbate (r = 0.518**) and other related parameters. The correlation values are shown in Table 3. Similarly the correlation between Cd-concentration with other related parameters were also assessed shown in Table 3 .
Discussion
The present study indicated that Cd has a toxic effect on host plant growth and nodule development of Vigna radiata. The concentration Fe/Cd analyzed in experimental soils indicated that Fe has greater effect on alleviating Cd stress by indicating that Fe content highly decreased in −Fe/−Cd plants while increased in −Fe/+Cd plants (Fig. 1a) whereas Cd content was negligible in control plants which increased in −Fe/−Cd plants however, decreased in +Fe/+Cd plants (Fig. 1b). These results were consistent with previous results of barley roots under S and Fe deficiency in relation to Cd-exposure (Astolfi et al. 2012). Cd-exposure reduced significantly leaf, root length, chlorophyll content of host plant, also decreased the number and biomass of nodules (Table 1). The negative effects of Cd-exposure on these parameters appeared to be much less in the plants fed with sufficient Fe (+Fe/+Cd) than in deficient Fe (−Fe/+Cd) and non-exposed cadmium (−Fe/Cd). The toxic effects of Cd induced oxidative stress as evidenced by the increased lipid peroxidation level (Fig. 3a). Lipid peroxidation has been considered as one of serious phytotoxic consequences of reactive oxygen species (ROS) generation (Ali et al. 2005; Lee et al. 2009). In this study, Cd-exposure resulted in the reduction in total glutathione and ascorbate content under Fe-deficient condition (Fig. 3b, c) and the activity of antioxidative enzymes (APX, SOD, CAT, GR) (Fig. 4), whereas they were significantly increased under Fe-sufficient condition whereas, less effect was observed in non-exposed cadmium with Fe deficiency (−Fe/−Cd). Several reviews have been published on different aspects of ascorbate and glutathione, ranging from their biosynthesis (Smirnoff et al. 2001; Potters et al. 2002), their roles in transport system (Horemans et al. 2000) and stress defense (Noctor and Foyer 1998) in plants. It has been generally established that glutathione and ascorbate play a prominent role in non-enzymatic mechanism to prevent oxidation of cellular compounds (Noctor and Foyer 1998) and involve in the induction of enzymes and gene expression (Alscher et al. 1987). In this study, the responses of glutathione and ascorbate to Cd/Fe combined treatment were well consistent with those of antioxidative enzymes. Indeed, APX, SOD, CAT, and GR activity in +Fe/+Cd plants significantly increased compared to controls (Fig. 4). In white clover, ascorbate–glutathione pathway was highly induced with high activation of APX without visible injury or lipid peroxidation during the early period of drought stress (Lee et al. 2007). Similarly, the water deficit-induced activation of SOD and CAT increased during the first 14 days (Lee et al. 2009). In particular, the increase in SOD activity was high during this period. This activation of SOD might be associated with it having an effective role in protection against water-deficit induced oxidative stress. Foyer and Noctor (2000), for instance, concluded that as SOD catalyzes the dismutation of superoxide radicals to H2O2 and O2, it is perhaps the most important enzyme in cellular defense because it directly modulates the amounts of O2·−and H2O2. Similarly it has been shown that salt stress induces an increase in SOD activity, and this has been frequently correlated with salt tolerance (Sreenivasulu et al. 2000; Sudhakar et al. 2001). A high activation of CAT in +Fe/+Cd plants (Fig. 4c) reflects that the H2O2 produced by SOD would in turn be scavenged mostly by CAT. The elevated activity of GR observed in −Fe/−Cd and more severe in +Fe/Cd plants may be also ascribable to the increased demand for reduced glutathione as a result of increased activity glutathione peroxidase. Glutathione is involved as a substrate for glutathione peroxidase and is therefore necessary for the removal of lipid peroxidases because GR reduces the oxidized glutathione which is generated by glutathione peroxidase (Potters et al. 2002; Ali et al. 2005). The observed increase in glutathione and ascorbate in +Fe/Cd plants without change (24 h) or slight decrease (72 h) in lipid peroxidation level compared to controls suggested that the plants were still under mild intensity of stress so that they were capable to increase substrates and enzymes activity associated with ROS scavenging pathways.
In contrast, Cd exposure under Fe-deficient condition (−Fe/+Cd) significantly decreased glutathione and ascorbate concentration (Fig. 3b, c) along with the decrease in APX, SOD, CAT and GR activity (Fig. 4) with a concomitant increase of lipid peroxidation. However, in non-exposed cadmium along with Fe-deficiency (−Fe/−Cd) the effect was less as compared to −Fe/+Cd plants which indicates Fe is essential for alleviating toxic effects of cadmium.
Cd-exposure under Fe-deficient condition enhanced the intensity of oxidative stress as evidence given by twofold higher level of lipid peroxidation compared to control (Fig. 3a). It could be suggested that Cd-exposure under Fe-deficient condition resulted in an over production of ROS which lead to oxidative injury such as membrane lipid peroxidation, protein oxidation, enzyme inhibition and DNA and RNA damage (Qadir et al. 2004) because Fe is important for expression of proteins which lead to conversion of singlet oxygen into hydrogen peroxide and finally into water (Qureshi et al. 2010).
In −Fe/+Cd plants, the severe reduction in ascorbate and glutathione (Fig. 3b, c) might be due to glutathione depletion which in turn leads to a subsequent reduction in the ascorbate–glutathione cycle (Gomes-Júnior et al. 2006) and least effect was observed in −Fe/−Cd plants. Indeed, the decrease in the activity of APX and GR, antioxidative enzymes using glutathione and ascorbate as substrate, was more prominent compared with that of SOD and CAT (Fig. 4). It has been well known that CAT and APX are important enzymes in root nodules and susceptible to Fe because they are heme containing enzymes (Deakin and Broughton 2009). At 72 h after Cd-exposure, the activity of APX and CAT in −Fe/+Cd plants decreased by 64 and 30 %, respectively, compared to controls (Fig. 4a, c) and less affect was observed in −Fe/−Cd plants. These decreases in APX and CAT activity well indicate that the plants failed to meet the demand of Fe for the formation of heme group and also a non-heme iron atom. Similarly, the water deficit-induced activation of SOD, CAT and APX failed to increase or in some cases slightly decreased during injurious period of drought stress (Lee et al. 2009). Thus SOD-CAT-APX antioxidant system in severe stress intensity might not be effective in scavenging ROS generated by stress. Indeed, the present data clearly indicated that −Fe/+Cd plants suffered more severe intensity of oxidative stress compared with −Fe/−Cd and +Fe/+Cd ones so that they failed to retain non-enzymatic and enzymatic mechanism to prevent harmful effects of ROS on membrane integrity and growth. Taken together, it could be concluded that Fe efficiently alleviates Cd-induced oxidative damage by the enhancement of antioxidant substrates (Fig. 3b, c) and antioxidative enzymes activity compared to control (Fig. 4).
This endurance to Cd toxic impact under Fe-sufficient condition might be explicated by a role of Fe in terms of fulfilling the requirements of iron a metal ligand for antioxidant enzymes as well as serving as binding force between proteins to form multi-protein complexes. Indeed, Cd exposure resulted in a reduction of protein concentration in root nodules. The negative effect of Cd-exposure was much less in Fe-sufficient than that in Fe-deficient condition (Fig. 2a). This might be due to the induction of specific metal binding proteins called metallothioneins and phytochelatins which mitigate the Cd stress involving sulforhydral groups (Benavides et al. 2005). Further, Cd-exposure resulted in significant decrease in leghemoglobin and ferritin concentration in nodules (Fig. 2b, c). This suggests that Cd exposure causes breakdown of bacterial proteins and flavonoids which help the root hairs in curling and formation of nodules (Balestrasse et al. 2004). Fe-deficiency has often resulted in reduction of nodulation and low formation of active nitrogenase, since nodules contain nitrogenase which requires Fe-Mo. It has been well documented that leghemoglobin is involved in N2-fixation in root nodules (Qureshi et al. 2010; Balestrasse et al. 2004). The oxygenated form of leghemoglobin can undergo slow autoxidation to the ferric form (metLb) giving rise to superoxide radicals and hence H2O2 by dismutation of singlet oxygen (Lodwig et al. 2003). Thus, Cd-induced oxidative stress severely restricts the activity of nitrogen fixing organs, since leghemoglobin is most important protein for the formation of nod genes (nif) (Balestrasse et al. 2004). Under drought conditions the content of leghemoglobin has been partially declined in alfalfa nodules (Van der Mark et al. 1981). These may be due to adverse effect on bacteriods which provides energy to nodules for nitrogen fixation. The reduction of leghemoglobin caused by Cd-exposure under Fe-deficient was remarkably recovered under Fe-sufficient condition (Fig. 2b). Furthermore, Cd-exposure decreased ferritin concentration in nodules much more severely under Fe-deficient (−92 % compared to control) than under Fe-sufficient condition (−33 %) (Fig. 2c) and −Fe/−Cd conditions. This suggested that Fe-deficiency aggravates Cd-induced breakdown of ferritin subunits. It has been well documented that ferritin involves in storage of Fe and protection of cells (Vincent et al. 2007). The results clearly indicate that Fe has a potential role in alleviating the negative effects of Cd-exposure on the formation, vitality and function of nodules by protecting the cells against ROS. The correlation curves between Fe/Cd and other related parameters (Table 3) assessed showed that Fe is important for physiological processes and proves that Fe plays an important role in mitigating Cd stress as r values in case of correlation between Fe with soluble protein, leghemoglobin and ferritin content were (P ≤ 0.001), whereas, (P ≤ 0.01) in terms of Cd-concentration. Indeed microscopic investigation of root nodules revealed that Cd-exposure altered the ultra-structure of root nodules (Fig. 5). In the plants under Fe-deficient condition (−Fe/+Cd), the number of bacteriods and cortical cell divisions was eventually ceased up (Fig. 5c) and distorted and ruptured shape of cell wall were observed (Fig. 5g). When compared with −Fe/+Cd plants, bacteroid and cell numbers relatively increased in Fe sufficient plants (+Fe/+Cd) (Fig. 5b) and deformation of cell wall remarkably alleviated even though distorted parts were still observed (Fig. 5f) and −Fe/−Cd plants (Fig. 5d, h). These suggested that Fe plays an efficient role in growth and development of root-nodules by protecting physical structure against Cd-toxic impact and in mitigating the alteration of nodulated genes (nod and nif) (Balestrasse et al. 2004) which are responsible for the formation of symbiosomes.
Recent advances in proteomic technologies provide the ability to discern sites of in vivo protein phosphorylation events in plant tissue in a high throughput manner by utilizing decision tree-driven tandem MS (Swaney et al. 2008). Several studies have been made on the root nodules of leguminous plants as a model plant Medicago trancatula (Larrainzar et al. 2007) and other species under different abiotic stress to identify the actual mechanism of host-pathogen relationship in which stress-responsive proteins are involved. Among twenty differentially proteins, eleven proteins responsive to combined Fe/+Cd impact were identified (Table 2). Large number of unknown and hypothetical proteins has been often reported in the root nodules under abiotic stress (Usadel et al. 2005; Thimm et al. 2004). Our study surprisingly also observed number of some unknown and hypothetical proteins (Table 2), although the reason remains unclear. This could be due to the fact that in root nodules most of the high abundant and detectable proteins have been characterized to be suitable for physiological processes. Furthermore, our proteomic analysis of root nodules identified the proteins involving in RNA metabolism and storage of grains for long shelf as classified and described by Beven et al. (1998). Despite the evidence that root-nodule proteins were differentially expressed by the level of Fe under Cd-stressed condition, further trascriptomic works and metabolites profiling in the nodule organelles such as mitochondria, plastids, peroxisomes will be required since the proteomic analysis revealed abundant number of hypothetical and unknown proteins.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ali MB, Hahn EJ, Paek KY (2005) Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol Biochem 43:213–223
Alscher R, Bower J, Zipfel W (1987) The basis for different sensitivities of photosynthesis to SO2 in two cultivars of pea. J Exp Bot 38:99–108
Alvarez S, Berla BM, Sheffield J, Cahoon RE, Jez JM, Hicks LM (2009) Comprehensive analysis of the Brassica juncea root proteome in response to cadmium exposure by complementary proteomic approaches. Proteomics 9:2419–2431
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:555–570
Aronon JD, Israelstam GF (1979) Copper enzymes in isolated chloroplast oxidase in Beta vulgaris. Plant Physiol 42:287–292
Astolfi S, Zuchi S, Neumann G, Cesco S, Sanita di Toppi L, Pinton R (2012) Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation J Exp Bot. doi:10.1093/jxb/err344
Balestrasse KB, Gardey L, Galleego SM, Tomaro ML (2004) Response of antioxidant defense system in soybean nodules and roots subjected to cadmium stress. Aust J Plant Physiol 28:497–504
Benavides MP, Susana MG, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17(1):21–34
Beven M et al (1998) Analysis of 1.9 Mb contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391:485–493
Clement JC, Shrestha J, Ehrenfeld JG, Jaffe PR (2005) Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol Biochem 33:2323–2328
Deakin WJ, Broughton WJ (2009) Symbiotic use of pathogen strategies: rhizobial protein secretion. Nature Rev Biol 7:312–320
Dhindsa RH, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Fagioni MD, Amici GM, Timperio AM, Zolla L (2009) Proteomic analysis of multi protein complexes in the thylakoid membrane upon cadmium treatment. J Proteome Res 8:310–326
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
Gomes-Júnior RA, Moldes CA, Delite FS, Pompeu GB, Gratão PL, Mazzafera P, Lea PJ, Azevedo RA (2006) Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65:1330–1337
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, New York
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hiscox JD, Israclstam GF (1979) A method for interaction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Horemans N, Foyer CH, Potters G, Asard H (2000) Ascorbate functions and associated transport systems in plants. Plant Physiol Biochem 38:531–540
Krouma A, Drevon JJ, Abdelly C (2006) Genotypic variation of nitrogen fixing common bean (Phaseolus vulgaris L.) in response to iron deficiency. J Plant Physiol 163:1049–1100
La Rue TA, Child JJ (1979) Sensitive flurometric assay for leghemoglobin. Ann Biochem 92:11–15
Larrainzar E, Wienkoop S, Weckwerth W, Ladrera R, Arrese-Igor C, González EM (2007) Medicago truncatula root nodule proteome analysis reveals differential plant and bacteroid responses to drought stress. Plant Physiol 144:1495–1507
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach leaves (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochem J 210(899):903
Lee BR, Kim KY, Jung WJ, Avice JC, Ourry A, Kim TH (2007) Peroxidases and lignification in relation to the intensity of water deficit stress in white clover (Trifolium repens L.). J Exp Bot 58:1271–1279
Lee BR, Jung WJ, Jin YL, Avice JC, Ourry A, Kim TH (2009) Water deficit-induced oxidative stress and the activation of antioxidative enzymes in white clover leaves. Biol Plant 53:505–510
Lodwig EM, Hosie AHF, Bourdès A, Findlay K, Allaway D, Karunakaran R, Downie JA, Poole PS (2003) Amino-acid cycling drives nitrogen fixation in the legume-rhizobium symbiosis. Nature 422:722–726
Maroco JP, Rodrigues ML, Lopes C, Chaves MM (2002) Limitations to photosynthesis in grapevine under drought-metabolic and modelling approaches. Funct Plant Physiol 29:1–9
Molloy MP, Herbert BR, Walsh BJ, Tyler MI, Traini M (1998) Extraction of membrane proteins by differential solubilization for separation using two dimensional gel electrophoresis. Electrophoresis 19:837–844
Muneer S, Ahmad J, Bashir H, Moiz S, Qureshi MI (2011) Studies to reveal importance of Fe for Cd tolerance in Brassica juncea. Int J Appl Biotech Biochem 1(3):321–338
Nakano M, Nobuta K, Vemaraju K, Tej SS, Skogen JW, Meyers BC (2006) Plant MPSS databases: signature-based transcriptional resources for analyses of mRNA and small RNA. Nucleic Acids Res 34:731–735
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
O’ Hara GW, Boonkerd N, Dilworth MJ (1998) Mineral constraints to nitrogen fixation. Plant Soil 108:98–110
Pinto AP, Mota AM, de varennes A, Pinto FC (2004) Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Plant Sci Total Environ. 326:239–247
Potters G, De Gara L, Assad H, Horemans (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40:537–548
Qadir S, Qureshi MI, Javed S, Abdin MZ (2004) Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd-stress. Plant Sci 167:1171–1181
Qureshi MI, Amici GMD, Fagioni M, Rinalducci S, Zolla L (2010) Iron stabilizes thylakoid protein-pigment complexes in Indian mustard during Cd-phytoremediation as revealed by BN-SDS-PAGE and ESI-MS/MS. J Plant Physiol 167:761–770
Ragland M, Theil EC (1993) Ferritin (mRNA, protein) and iron concentrations during soybean nodule development. Plant Mol Biol 21:55–560
Rao MV (1992) Cellular detoxification mechanisms to determine age dependent injury in tropical plant exposed to SO2. J Plant Physiol 140:733–740
Roskams AJI, Connors JR (1994) Iron, transferring and ferritin in the rats brain during development and aging. J Neurochem 63:709–716
Roth UE, Von RP, Clemens S (2006) Proteome changes in Arabidopsis thaliana roots upon exposure to Cd. J Exp Bot 57:4003–4013
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 4:105–130
Sárvári É, Fodor F, Cseh E, Varga A, Záray GY, Zolla L (1999) Relationship between changes in ion content of leaves and chlorophyll–protein composition in cucumber under Cd and Pb stress. Z Naturforsch 54c:746–753
Siedlecka A, Krupa Z (1999) Cd/Fe interaction in higher plants—its consequences for the photosynthetic apparatus. Photosynthetica 36:321–331
Smirnoff N, Conklin PL, Loewus FA (2001) Biosynthesis of ascorbic acid in plants: a renaissance. Ann Rev Plant Physiol Plant Mol Biol 52:437
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt sensitive seedlings of foxtail millet (Setaria italica) Physiol. Plant 109:435–442
Sudhakar C, Lakshmi A, Giridara Kumar A (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Swaney DL, McAlister GC, Coon JJ (2008) Decision tree–driven tandem mass spectrometry for shotgun proteomics. Nature 5:959–964
Tang C, Robson AD, Dilworth MJ (1991) The role of iron in nodulation and nitrogen fixation in Lupin anustifolius L. is sensitive to iron deficiency. New Phytol 117:243–250
Thimm O, Blasing O, Gibon Y, Nagel A, Meyer S, Kruger P, Selbig J, Muller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
Usadel B, Nagel A, Thimm O, Redestig H, Blaesing OE, Palacios-Rojas N, Selbig J, Hannemann J, Piques MC, Steinhauser D et al (2005) Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol 138:1195–1204
Van der Mark F, de Lange T, Bienfait HF (1981) The role of ferritin in developing primary bean leaves under various light conditions. Planta 153:338–342
Vanoni MA, Curti B (2005) Structure-function studies on the iron sulfur flavoenzymes glutamate synthetase: an unexpectedly complex self-regulated enzyme. Arch Biochem. Biophy. 433:193–211
Vincent D, Ergul A, Bohlman MC, Tattersall EA, Tillet RL, Wheatley MD (2007) Proteomic analysis reveals differences between Vitis venifera L. cv. Chardonnay and CV. Cabernet sauvignon and their response to water deficit and salinity. J Exp Bot 5B:1873–1893
Acknowledgments
This study has been supported by UGC (University grants commission) and DST (Department of Science and Technology), Govt. of India.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Muneer, S., Kim, T.H. & Qureshi, M.I. Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata . Plant Growth Regul 68, 421–433 (2012). https://doi.org/10.1007/s10725-012-9731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9731-1