Abstract
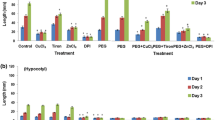
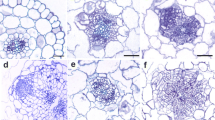
The production of reactive oxygen species (ROS) plays important roles in the life cycle and in the stress response and defence mechanisms of plants. Various enzyme systems are involved in the formation of ROS in the apoplast, including plasmalemma NADPH oxidase and apoplastic peroxidases. The production of O ·−2 and apoplastic peroxidase and exogenous NADH oxidation activities are all strongly dependent on the age of roots—the younger the root, the greater the activity. Apoplastic production of ROS is shown in the root by using specific histochemical probes, this ROS production is growing zone dependent. In the present study, using olive seedlings, differences were also observed between cultivars, especially in O ·−2 production by the Verdial cultivar which was well above that of other cultivars studied. In all the cultivars, treatment of roots with methyl jasmonate (MeJA) or methyl salicylate (MeSA) increased O ·−2 production. Similar results were observed for peroxidase activity, but not for the oxidation of exogenous NADH which was either unaffected (MeJA) or even partially inhibited (MeSA). A conclusion was that MeJA or MeSA induced apoplastic production of ROS does not use exogenous NADH. Treatment with diphenylene iodonium (DPI) reduced the formation of O ·−2 , but affected neither peroxidase nor NADH oxidation activities. Cyanide inhibited O ·−2 production and peroxidase and NADH oxidation activities. Treatment with MnCl2 had a strong stimulatory effect on peroxidase and NADH oxidation activities, but much less on O ·−2 production. Finally, azide greatly reduced all activities, but especially O ·−2 production. Together, these results indicate a relationship between oxidative activities and the processes of root growth, and that those activities are also dependent on the cultivar, as well as an involvement of peroxidases and plasmalemma NADPH oxidase in apoplast ROS production which is sensitive to DPI, azide, and cyanide but relatively insensitive to MnCl2, while exogenous NADH oxidation is linked to peroxidase activity.





Similar content being viewed by others
Abbreviations
- DMAB:
-
3-Dimethylaminobenzoic acid
- DPI:
-
Diphenylene iodonium
- EDTA:
-
Ethylenediaminetetraacetic acid
- MBTH:
-
3-Methyl-2-benzothiazolinone hydrazone
- MeJA:
-
Methyl jasmonate
- MeSA:
-
Methyl salicylate
- PM:
-
Plasma membrane
- RBOH:
-
Respiratory burst oxidase homologue
- ROS:
-
Reactive oxygen species
- ECPOX:
-
Extracellular peroxidases
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 109:1247–1257
Baker CJ, Deahl K, Domek J, Orlandi W (1998) Oxygen metabolism in plant/bacteria interations: effect of DPI on the pseudo-NAD(P)H oxidase activity of peroxidase. Bichem Biophys Res Commun 252:461–464
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component systems. J Exp Botany 53:1367–1376
Cañas LA, Carramolino L, Vicente M (1987) Vegetative propagation of the olive tree from in vitro cultured embryos. Plant Sci 50:85–90
Chen S-X, Schopfer P (1999) Hydroxyl radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem 260:726–735
Dangl JL, Dietrich RA, Richberg MH (1996) Death don′t have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8:1793–1807
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Garrido I, Espinosa F, Córdoba-Pedregosa MC, González-Reyes JA, Alvarez-Tinaut MC (2003) Redox related peroxidative responses evoked by Methyl-Jasmonate in axenically cultured aeroponic sunflower (Helianthus annuus, L.) seedling roots. Protoplasma 221:79–91
Garrido I, Espinosa F, Álvarez-Tinaut MC (2009) Oxidative defence reactions in sunflower roots induced by methyl-jasmonate and methyl-salicylate and their relations with calcium signaling. Protoplasma 237:27–39
Grant JJ, Yun B-W, Loake GJ (2000) Oxidative burst and cognate redox signalling reported by luciferase Imaging: identification of a signal network that functions independently of ethylene, Sa and Me-JA but is dependen ton MAPKK activity. Plant J 24:569–582
Horváth E, Szalai G, Janda T (2007) Induction of abiotic stress tolerante by salicylic acid signaling. J Plant Growth Regul 26:290–300
Kawano T (2003) Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 21:829–837
Lizskay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217:658–667
Lizskay A, van der Zalm E, Schopfer P (2004) Productioin of reactive oxygen intermediates (O ·−2 , H2O2 and.OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Maksimovic J, Maksimovic V, Zivanovic B, Sukalovic H-TV, Vuletic M (2008) Peroxidase activity and phenolic compounds content in maize root and leaf apoplast, and their association with growth. Plant Sci 175:656–662
Miché L, Battistoni F, Gemmer S, Belghazi M, Reinnhold-Hurek B (2006) Upresgulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol Plant Microbe Interact 19:502–511
Mika A, Minibayeba F, Beckett R, Lüthje S (2004) Possible functions of extracellular peroxidases in stress-induced generation and detoxification of active oxygen species. Phytochem Rev 3:173–193
Minibayeba FV, Gordom LK, Kolesnikov OP, Chasov AV (2001) Role of extracellular peroxidase in the superoxide production by wheat root cells. Protoplasma 217:125–128
Minibayeba FV, Mika A, Lüthje S (2003) Salicylic acid changes the properties of extracellular peroxidase activity secreted from wounded wheat (Triticum aestivum L.) roots. Protoplasma 221:67–72
Misra HR, Fridovich I (1972) The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem 24:188–192
Ngo TT, Lenhoff HM (1980) A sensitive and versatile chromogenic assay for peroxidase-coupled reactions. Anal Biochem 105:389–397
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9(11):534–540
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolismo of pea (Pisum sativum L.) roots. Imaginf of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Rubinstein B, Stern AI, Stout RG (1984) Redox activity at the surface of oat root cells. Plant Physiol 76:386–391
Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD (2001) Jasmonic acid carboxylmethyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA 99:517–522
Staswick PE (1992) Jasmonate, genes and fragrant signals. Plant Physiol 99:804–807
Torres MA, Dangl JL, Jones JDG (2006) Reactive oxygen species signalling in response to pathogens. Plant Physiol 141:373–378
Wojtaszek B (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322:681–692
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garrido, I., Caraballo-Sánchez, A.M., Llerena, J.L. et al. Oxidative reactions in axenically seedling roots of olive (Olea europaea, L.). Plant Growth Regul 68, 203–210 (2012). https://doi.org/10.1007/s10725-012-9708-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9708-0