Abstract
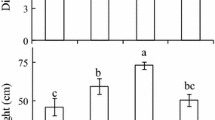
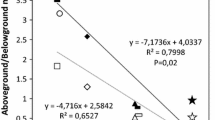
The possible involvement of salicylic acid (SA) in a typical growth response of plants to shade light was investigated using the model system Stellaria longipes L. Goldie. The prairie (shade) ecotype of S. longipes is from foothills grassland habitat where it grows under shrubs or among taller grasses. The plants of this ecotype responded, as expected, with increased growth under lower red to far-red (R/FR) ratio and reduced photosynthetically active radiation (PAR). By contrast, the alpine (sun) ecotype is from an open sunny habitat, where canopy shade is a non-factor. The plants of this ecotype failed to respond with increased growth under a lower R/FR ratio, but had increased growth under a reduced PAR level. To examine the possible role of SA in shade light-mediated growth, the two main components of shade light, R/FR ratio and PAR, were uncoupled, and a series of experiments were performed by measuring the endogenous SA content and the plant response to exogenous SA concentrations. Contrary to the alpine plants, the prairie plants had increased endogenous SA content and higher shoot biomass accumulation under a low R/FR ratio treatment compared with normal or high R/FR ratios. Both alpine and prairie plants responded to a low PAR treatment with a decrease in endogenous SA content and an increase in shoot biomass accumulation, but the magnitude of this response was higher in prairie plants. Based on the results of this study, we conclude that shoot SA content is differentially regulated by both R/FR ratio and PAR signals, and SA may contribute, in ecotype specific manner, to growth changes in plants subjected to changing light environments.






Similar content being viewed by others
References
Ballare CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4:97–102
Beall FD, Yeung EC, Pharis RP (1996) Far-red light stimulated internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can J Bot 74:743–752
Emery RJN, Chinnappa CC, Chmielewski JG (1994) Specialization, plant strategies, and phenotypic plasticity in populations of Stellaria longipes along an elevational gradient. Int J Plant Sci 155:203–219
Gaskin P, MacMillan J (1991) GC-MS of the gibberellins and related compounds. Methodology and a library of spectra. University of Bristol, Bristol (Cantock’s Enterprises)
Genoud T, Buchala AJ, Chua NH, Metraux JP (2002) Phytochrome signalling modulates the SA perceptive pathway in Arabidopsis. Plant J 31:87–95
Gunes A, Inal A, Alpaslan M, Cicek N, Guneri E, Eraslan F, Guzelordu T (2005) Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.). Arch Agron Soil Sci 51:687–695
Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002) Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115:428–441
Koshioka M, Takeno K, Beall FD, Pharis RP (1983) Purification and separation of gibberellins from their precursors and glucosyl conjugates. Plant Physiol 73:398–406
Kurepin LV, Walton LJ, Reid DM, Pharis RP, Chinnappa CC (2006a) Growth and ethylene evolution by shade and sun ecotypes of Stellaria longipes in response to varied light quality and irradiance. Plant, Cell Environ 29:647–652
Kurepin LV, Pharis RP, Reid DM, Chinnappa CC (2006b) Involvement of gibberellins in the stem elongation of sun and shade ecotypes of Stellaria longipes that is induced by low light irradiance. Plant, Cell Environ 29:1319–1328
Kurepin LV, Emery RJN, Pharis RP, Reid DM (2007a) The interaction of light quality and irradiance with gibberellins, cytokinins and auxin in regulating growth of Helianthus annuus hypocotyls. Plant, Cell Environ 30:147–155
Kurepin LV, Emery RJN, Pharis RP, Reid DM (2007b) Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: putative roles for plant hormones in leaf and internode growth. J Exp Bot 58:2145–2157
Kurepin LV, Shah S, Reid DM (2007c) Light quality regulation of endogenous levels of auxin, abscisic acid and ethylene production in petioles and leaves of wild type and ACC deaminase transgenic Brassica napus seedlings. Plant Growth Regul 52:53–60
Kurepin LV, Walton LJ, Reid DM (2007d) Interaction of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regul 51:53–61
Kurepin LV, Emery RJN, Chinnappa CC, Reid DM (2008) Light irradiance differentially regulates endogenous levels of cytokinins and auxin in alpine and prairie genotypes of Stellaria longipes. Physiol Plant 134:624–635
Kurepin LV, Walton LJ, Reid DM, Chinnappa CC (2010) Light regulation of endogenous salicylic acid levels in hypocotyls of Helianthus annuus seedlings. Botany 88:668–674
Lee SH, Reid DM (1997) The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Can J Bot 78:501–508
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Reid DM, Sheffer MG, Pierce RC, Bezdicek DF, Linzon SN, Revven T, Spenser MS, Vena F (1985) Ethylene in the environment: scientific criteria for assessing its effects on environmental quality. National Research Council of Canada, Ottawa, publication 22497
Smith H (2000) Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407:585–591
Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D (2005) Reaching out of the shade. Cur Opin Plant Biol 8:462–468
Zobel RW, Robert LW (1978) Effect of low concentration of ethylene on cell division and cell differentiation in lettuce pit explants. Can J Bot 56:987–990
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurepin, L.V., Walton, L.J., Hayward, A. et al. Shade light interaction with salicylic acid in regulating growth of sun (alpine) and shade (prairie) ecotypes of Stellaria longipes . Plant Growth Regul 68, 1–8 (2012). https://doi.org/10.1007/s10725-012-9686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9686-2