Abstract
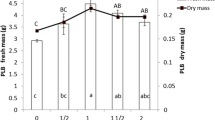
An in vitro culture procedure was established to induce protocorm-like bodies (PLBs) from leaf segments of the Phalaenopsis bellina (Rchb.f.) Christenson directly from epidermal cells without intervening callus on ½ strength modified Murashige and Skoog (MS) (in Physiol Plant 15:473–497, 1962) medium supplemented with 1-Naphthaleneacetic acid (NAA; 0, 0.1, 1 mg/l) and Thidiazuron (TDZ; 0, 0.1, 1, 3 mg/l). The best response was established at 3 mg/l TDZ which induced 78% of leaf segments to form a mean number of 14 PLBs per explant after 16 weeks of culture. No PLBs were found when leaf segments were cultured on ½ strength modified MS media supplemented with 0.1 and 1 mg/l NAA. The best induction percentage for auxin: cytokinin combination was at the combination of NAA and TDZ at 1.0 and 3.0 mg/l which gave 72% induction with 9 PLBs per explant. Semi-solid ½ strength MS and liquid Vacin and Went (VW) (in Bot Gaz 110:605–613, 1949) medium were used in order to find the highest survival and number of PLBs proliferation after 3 months in culture. Half strength MS showed an average of 9 PLBs in comparison with VW with an average of 5.3 PLBs per explants. Histological observations revealed that the regenerated PLBs were generally formed from the epidermal layers of the posterior regions of the leaf segments. Scanning electron micrograph of PLBs showed the origin of newly formed PLB from the peripheral region of leaf segments.






Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog media
- VW:
-
Vacin and Went media
- NAA:
-
1-Naphthaleneacetic acid
- TDZ:
-
1-phenyl-3-(1, 2, 3-thiadiazol-5-yl)-urea
- PLB:
-
Protocorm like body
References
Arditti J, Ernst R (1993) Micropropagation of orchids. Wiley Publishers, New York
Bhadra SK, Hossain MM (2004) Induction of embryogenesis and direct organogenesis in Micropera pallida Lindl., an epiphytic orchid of Bangladesh. J Orchid Soc India 18:5–9
Chen JT, Chang WC (2001) Effects of auxins and cytokinins on direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramsey’. Plant Growth Regul 34:229–232
Chen JT, Chang WC (2004) Induction of repetitive embryogenesis from seed-derived protocorms of Phalaenopsis amabilis var. formosa Shimadzu. In Vitro Cell Dev Biol 40:290–293
Chen JT, Chang WC (2006) Direct somatic embryogenesis and plant regeneration from leaf explants of Phalaenopsis amabilis. Biol Plant 50:169–173
Chen Y, Piluek C (1995) Effects of thidiazuron and N6- benzylaminopurine on shoot regeneration of Phalaenopsis. Plant Growth Regul 16:99–101
Chen JT, Chang C, Chang WC (1999) Direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramsey’ and subsequent plant regeneration. Plant Cell Rep 19:143–149
Chen YC, Chang C, Chang WC (2000) A reliable protocol for plant regeneration from callus culture of Phalaenopsis. In Vitro Cell Dev Biol Plant 36:420–423
Chugh S, Guha S, Rao RU (2009) Micropropagation of orchids: a review on the potential of different explants. Sci Hortic 122:507–520
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Gersterberger P, Leins P (1978) Rasterelektronenmikroskopische Untersuchungen an Blütenknospen vonPhysalis philadelphica (Solanaceae)-Anwendung einer neuen Präparationsmethode. Ber Deutsch Bot Ges 91:381–387
Hew CS, Yong JWH (2004) The physiology of tropical orchids in relation to the industry. New Jersey World Scientific Pub Co. Inc., NJ
Hsiao YY, Tsai WC, Kuoh CS, Huang TH, Wang HC, Wu TS, Leu YL, Chen WH, Chen HH (2006) Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce monoterpene biosynthesis pathway. BMC Plant Biol 6:14
Huetteman CA, Precee JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Islam MO, Rahman ARMM, Matsui S, Prodhan AKMA (2003) Effects of complex organic extracts on callus growth and PLB regeneration through embryogenesis in the Doritaenopsis orchid. Jpn Agric Res Q 37(4):229–235
Jensen WA (1962) Botanical histochemistry. Freeman, San Francisco
Lin CC (1986) In vitro culture of flower stalk internodes of Phalaenopsis and Doritaenopsis. Lindleyana 1:158–163
Mahmood M, Chew YC (2008) Agrobacterium-mediated genetic transformation of Phalaenopsis bellina using GFP and GUS reporter genes. Pertanika J Sci & Technol 16(2):129–139
Mayr H (1998) Orchid names and their meaning. Koeltz Scientific Books, Koenigstein
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Nagl W, Rücker W (1976) Effects of phytohormones on thermal denaturation profiles of Cymbidium DNA: indication of differential DNA replication. Nucleic Acids Res 3:2033–2039
Nash N (2003) Phalaenopsis primer: a beginner’s guide to growing moth orchids. Orchids 72:906–913
Park SY, Murthy HN, Paek KY (2002a) Rapid propagation of Phalaenopsis from floral stalk-derived leaves. In Vitro Cell Dev Biol Plant 38:168–172
Park SY, Yeung EC, Chakrabarty D, Paek KY (2002b) An efficient direct induction of protocorm-like bodies from leaf subepidermal cells of Doritaenopsis hybrid using thin-section culture. Plant Cell Rep 21:46–51
Riera-Lizarazu O, Mujeeb-Kazi A, William MDHM (1992) Maize (Zea mays L.) mediated polyhaploid production in some Triticeae using a detached tiller method. J GenetBreed 46:335–346
Saieed NT, Douglas GC, Fry DJ (1994) Induction and stability of somaclonal variation in growth, leaf phenotype and gas exchange characteristics of Poplar regenerated from callus culture. Tree Physiol 14:1–16
Shiau YJ, Nalawade SM, Hsia CN, Mulabacal V, Tsay H (2005) In vitro propagation of the Chinese medicinal plant, Dendrobium candidum Wall. Ex Lindl., from axenic nodal segments. In Vitro Cell Dev Biol Plant 41:666–670
Subramanium G, Taha RM (2003) Morphogenesis of Cymbidium atropurpureum in vitro. Malays J Sci 22:1–5
Talukder SK, Nasiruddin KM, Yesmin S, Hassan L, Begum R (2003) Shoot proliferation of Dendrobium orchid with BAP and NAA. J Biol Sci 3(11):1058–1062
Tanaka M, Sakanishi Y (1980) Clonal propagation of Phalaenopsis through tissue culture. In: Kashemsanta MRS (ed) Proceedings of the 9th world orchid conference, Bangkok, pp 215–221
Tanaka M, Hasegawa A, Goi M (1975) Studies on the clonal propagation of monopodial orchids by tissue culture. I. Formation of protocorm-like bodies from leaf tissues in Phalaenopsis and Vanda. J Jap Soc Hort Sci 44:47–58
Teob ES (1989) Orchids of Asia. Times Books International, Singapore, pp 125–134
Thammasiri K (2002) Preservation of seeds of some Thai orchid species by vitrification. In: Proceedings of the 16th world orchid conference, pp 248–251
Tokuhara K, Mii M (2003) Highly-efficient somatic embryogenesis from cell suspension cultures of Phalaenopsis orchids by adjusting carbohydrate sources. In Vitro Cell Dev Biol 39:635–639
Vacin E, Went F (1949) Some pH changes in nutrient solutions. Bot Gaz 110:605–613
Vajrabhaya M, Vajrabhaya T (1970) Culture of Rhynchostylis gigantea a monopodial orchid. Am Orchid Soc Bull 39:907–910
Ye XL, Cheng SJ, Wang FX, Qian NF (1988) Morphology of immature seeds and development in vitro of Dendrobium candidum. Acta Bot Yunnanica 10:285–290
Zhang QX, Fang YM (2005) Tissue culture and in vitro seedling and protocorm-like body examination of Dendrobium candidum. Acta Bot Boreali- Occidentalia Sinica 25:1761–1765
Zhao P, Wu F, Feng FS, Wand WJ (2008) Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum wall ex Lindl. In Vitro Cell Dev Bio Plant 44:178–185
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoddamzadeh, A.A., Sinniah, U.R., Kadir, M.A. et al. In vitro induction and proliferation of protocorm-like bodies (PLBs) from leaf segments of Phalaenopsis bellina (Rchb.f.) Christenson. Plant Growth Regul 65, 381–387 (2011). https://doi.org/10.1007/s10725-011-9611-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9611-0