Abstract
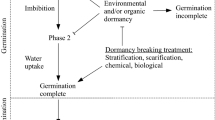
Many Disa species (80%) have not been germinated in vitro, fuelling conservation fears. Winter-rainfall species are typically germinable, whilst summer-rainfall species are exclusively intractable in vitro. We aimed to establish the in vitro germination requirements for previously ungerminated Disa species. Conventional asymbiotic seed culture protocols were reviewed and their efficacy in summer-rainfall Disa established. Mature seed was subjected to manipulations of media composition and viscosity, incubation temperature and illumination. Immature cultures were also established across four seed maturity classes. Four first-time germinations (D. cooperi, D. nervosa, D. pulchra and D. woodii) resulted from media with increased water availability. Germination rate (>12 weeks), percentage (<30%) and synchrony were not in accordance with values reported for winter-rainfall species (>80% germination in 8 weeks). Germination of immature seed under control conditions was similar to mature seed germination under modified conditions, but neither percentage approached the calculated germination potential (∼viability). Germination control in Disa is proposed as a trade-off between water availability and the presence of phyto-inhibitors in the environment of the embryo—two features typical of seeds exhibiting water-impermeable dormancy.
Similar content being viewed by others
References
Anderson AB (1990) Asymbiotic germination of seeds of some North American orchids. In: Sawyers CE (eds) North American native terrestrial orchid propagation and production. Chadds Ford Press, Pennsylvania, USA
Anderson AB (1991) Symbiotic and asymbiotic germination and growth of Spiranthes magnicamporum (Orchidaceae). Lindleyana 6:183–186
Arditti J (1967) Factors affecting the germination of orchid seeds. Bot Rev 33:24–29
Arditti J (1979) Aspects of the physiology of orchids. In: Wollhouse H (ed) Advances in botanical research 7. London, UK, Academic Press
Arditti J (1982) Orchid seed germination and seedling culture—a manual. In: Arditti J (ed) Orchid biology—reviews and perspectives II. New York, USA, Cornell University Press
Arditti J, Ernst R (1984) Physiology of germinating orchids seeds. In: Arditti J (ed) Orchid biology—reviews and perspectives III. New York, USA, Cornell University Press
Arditti J, Michaud JD, Healey PL (1979) Morphometry of orchid seeds. I. Paphiopedilum and native California and related species of Cypripedium. Am J Bot 66:1128–1137
Arditti J, Michaud JD, Healey PL (1980) Morphometry of orchid seeds. II. Native California and related species of Calypso, Cephalanthera, Corallorhiza and Epipactis. Am J Bot 67:347–360
Arditti J, Michaud JD, Oliva AP (1981) Seed germination of North American orchids. I. Native Californian and related species of Calypso, Epipactis, Goodyera, Piperia and Platanthera. Bot Gaz 142:442–453
Arditti J, Michaud JD, Oliva AP (1982a) Practical germination of North American and related orchids. I. Epipactis atrorubens, E. gigantea and E. helleborine. Am Orchid Soc Bull 51:162–171
Arditti J, Michaud JD, Oliva AP (1982b) Practical germination of North American and related orchids. II. Goodyera oblongifolia and G. tessellata. Am Orchid Soc Bull 54:859–866
Ballard WW (1987) Sterile propagation of Cypripedium reginae from seeds. Am Orchid Soc Bull 56:935–946
Ballard WW (1990) Further notes on Cypripedium germination. In: Sawyers CE (ed) North American native terrestrial orchid propagation and production. Chadds Ford Press, Pennsylvania, USA
Baskin CC, Baskin JM (1998) Seeds—ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego USA
Batty AL, Dixon KW, Brundrett M, Sivasithamparam K (2001a) Constraints to symbiotic germination of terrestrial orchid seed in a Mediterranean bushland. New Phytol 152:511–520
Batty AL, Dixon KW, Brundrett M, Sivasithamparam K (2001b) Long-term storage of mycorrhizal fungi and seed as a tool for the conservation of endangered western Australian terrestrial orchids. Aust J Bot 49:619–628
Beck SL (1999) Micropropagation of Acacia mearnsii De Willd. PhD Thesis, University of Natal, Pietermaritzburg
Bergman FJ (1995) The disinfection of orchid seed. Orchid Rev 103:217–219
Bergman FJ (1996) Orchid seed disinfection—the effect of pH and temperature. Orchid Rev 104:245–247
Bewley JD, Black M (1994) Seeds: physiology of development and germination. Plenum Press, New York, USA
Borris H (1969) Samenvermehrung und anzucht Europäischer erdorchideen. In: Proceedings of the 2nd European Orchid Congress. Paris, France
Burgeff H (1959) Mycorrhiza of orchids. In: Withner CL (ed) The orchids: a scientific survey. Ronald Press, New York, USA
Butcher D, Marlow SA (1989) Asymbiotic germination of epiphytic and terrestrial orchids. In: Pritchard HW (ed) Modern methods in orchid conservation: the role of physiology, ecology and management. Cambridge University Press, New York, USA
Chu C-C, Mudge KW (1994) Effects of prechilling and liquid suspension culture on seed germination of the yellow Lady’s slipper orchid (Cypripedium calceolus var. pubescens). Lindleyana 9:153–159
Clements MA (1982) Developments in the symbiotic germination of Australian terrestrial orchids. In: Stewart J, van der Merwe CN (eds) Proceedings of the 10th World Orchid Conference. South African Orchid Council, Johannesburg, South Africa
Clements MA, Muir H, Cribb PJ (1986) A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bull 41:437–445
Coke JL (1990) Aseptic germination and growth of some terrestrial orchids. In: Sawyers CE (ed) North American native terrestrial orchid propagation and production. Chadds Ford Press, Pennsylvania, USA
Collett A (1971) Notes on the growing of Disa uniflora. J Roy Hort Soc 96:358–361
Dalla Rosa M, Laneri U (1977) Modification of nutrient solutions for germination and growth in vitro of some cultivated orchids and for the vegetative propagation of Cymbidium cultivars. Am Orchid Soc Bull 46:813–820
De Pauw MA, Remphrey WR (1993) In vitro germination of three Cypripedium species in relation to time of seed collection, media and cold treatment. Can J Bot 71:879–885
Ernst R (1967) Effect of carbohydrate selection on the growth rate of freshly germinated Phalaenopsis and Dendrobium seed. Am Orchid Soc Bull 36:1068–1073
Ernst R, Arditti J, Healey PL (1971) Carbohydrate physiology of orchid seedlings II: hydrolysis and effects of oligosaccharides. Am J Bot 58:827–835
Fast G (1982) European terrestrial orchids (symbiotic and asymbiotic methods). In: Arditti J (ed) Orchid biology—reviews and perspectives II. Cornell University Press, New York, USA
Ford CS (1999) Cryopreservation of Pinus patula Scheide et Deppe embryogenic tissue. MSc Thesis, University of Natal, Pietermaritzburg, South Africa
Galunder R (1984) Perönliche erfahrungen bei der asymbiotischen aussat mit unterschiedlichen agar-agar-mengen. Die Orchidee 35:224–226
George EF (1993) Plant propagation by tissue culture I: the technology, 2nd edn. Exergetics Ltd, Edington, UK
Hadley G (1982) Orchid mycorrhiza. In: Arditti J (ed) Orchid biology—reviews and perspectives II. Cornell University Press, New York, USA
Harborne JB (1989) General procedures and measurement of total phenolics. In: Harborne JB (ed) Methods in plant biochemistry I: plant phenolics. Academic Press, London, UK
Harvais G (1972) The development and growth requirements of Dactylorhiza purpurella in asymbiotic cultures. Can J Bot 50:1223–1229
Harvais G (1973) Growth requirements and development of Cypripedium reginae in axenic culture. Can J Bot 51:327–333
Harvais G (1974) Notes on the biology of some native orchids of Thunder Bay, their endophytes and symbionts. Can J Bot 52:451–460
Harvais G (1980) Scientific notes on a Cypripedium reginae of Northwest Ontario, Canada. Am Orchid Soc Bull 49:237–244
Harvais G (1982) An improved culture medium for growing the orchid Cypripedium reginae axenically. Can J Bot 60:2547–2555
Harvais G, Hadley G (1967) The relation between host and endophyte in orchid mychorriza. New Phytol 66:205–215
Hendrickson LE (1951) Asymbiotic germination of orchids and some effects of vitamins on Thunia marshalliana. Sven Bot Tidskr 45:447–459
Hilton-Taylor C (1996a) Red data list of southern African plants. Strelitzia 4:1–117
Hilton-Taylor C (1996b) Red data list of southern African plants. I. Corrections and additions. Bothalia 26:177–182
Hilton-Taylor C (1997) Red data list of southern African plants. II. Corrections and additions. Bothalia 27:195–209
Ichihashi S, Yamashita M (1977) Studies on the media for orchid seed germination. I. The effects of balances inside each cation and anion group for the germination of Bletilla striata seeds. J Jpn Soc Hort Sci 45:407–413
Knudson L (1943) Nutrient solutions for orchid seed germination. Am Orchid Soc Bull 12:77–79
Knudson L (1946) A new nutrient solution for orchid seed germination. Am Orchid Soc Bull 15:214–217
LaCroix IF, LaCroix E (1997) African orchids in the wild and in cultivation. Timber Press, Portland, USA
LeRoux G, Barabé D, Vieth J (1997) Morphogenesis of the protocorm of Cypripedium acaule (Orchidaceae). Plant Syst Evol 205:53–72
Liddell RW (1944) Germinating native orchid seed. Am Orchid Soc Bull 12:344–345
Lindén B (1980) Aseptic germination of seeds of northern terrestrial orchids. Ann Bot Fenn 17:174–182
Lindén B (1992) Two new methods for pre-treatment of seeds of northern orchids to improve germination in axenic culture. Ann Bot Fenn 29:305–313
Linder HP, Kurzweil H (1999) Orchids of southern Africa. AA Balkema, Rotterdam, the Netherlands
Lindquist B (1960) The raising of Disa uniflora seedlings in Gothenburg. Am Orchid Soc Bull 34:317–319
Lucke E (1977) Naturstoffzusätze bei der asymbiotischen samenvermehrung der orchideen in vitro. Die Orchidee 28:185–191
Manning JC, van Staden J (1987) The development and mobilisation of seed reserves in some African orchids. Aust J Bot 35:343–353
Masuhara G, Katsuya K (1989) Effects of mycorrhizal fungi on seed germination and early growth of three Japanese terrestrial orchids. Sci Hort 37:331–337
Mead JW, Bulard C (1975) Effects of vitamins and nitrogen sources on asymbiotic germination and development of Orchis laxiflora and Ophrys shpegodes. New Phytol 74:33–40
Mead JW, Bulard C (1979) Vitamins and nitrogen requirements of Orchis laxiflora Lamk. New Phytol 83:129–136
Michel EE (2002) Asymbiotic propagation of tropical terrestrial orchid species. In: Clark J, Elliott WM, Tingley G, Biro J (eds) Proceedings of the 16th World Orchid Conference. Vancouver Orchid Society, Vancouver, Canada
Mitchell RB (1989) Growing hardy orchids from seeds at Kew. The Plantsman 11:152–169
Miyoshi K, Mii M (1995) Enhancement of seed germination and protocorm formation in Calanthe discolor (Orchideaceae) by NaOCl and polyphenol treatments. Plant Tiss Cult Lett 12:267–272
Miyoshi K, Mii M (1998) Stimulatory effects of sodium and calcium hypochlorite, pre-chilling and cytokinins on the germination of Cypripedium macranthos seed in vitro. Physiol Plant 102:481–486
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with Tobacco tissue cultures. Physiol Plant 15:473–497
Nakamura SI (1976) Atmospheric conditions required for the growth of Galeola septentrionalis seedlings. Bot Mag (Tokyo) 89:211–218
Nakamura SI (1982) Nutritional conditions required for the non-symbiotic culture of an achlorophyllous orchid Galeola septentrionalis. New Phytol 90:701–715
Oliva AP, Arditti J (1984) Seed germination of North American orchids. II. Native Californian and related species of Aplectrum, Cypripedium and Spiranthes. Bot Gaz 145:495–502
Pierik RLM, Steegmans HHM (1972) The effect of 6-benzylamino purine on growth and development of Cattleya seedlings grown from unripe seeds. Physiol Plant 68:228–234
Pritchard HW (1985) Growth and storage of orchid seeds. In: Tan KW (ed) Proceedings of the 11th World Orchid Conference. World Orchid Conference Inc, Miami, USA
Pritchard HW (ed) (1989) Modern methods in orchid conservation: the role of physiology, ecology and management. Cambridge University Press, New York, USA
Purves S, Hadley G (1976) The physiology of symbiosis in Goodyera repens. New Phytol 77:689–696
Raghavan V, Torrey JG (1964) Inorganic nitrogen nutrition of the seedlings of the orchid Cattleya. Am J Bot 51:264–274
Rasmussen HN (1992) Seed dormancy patterns in Epipactis palustris (Orchidaceae): requirements for germination and establishment of mycorrhiza. Physiol Plant 86:161–167
Rasmussen HN (1995) Terrestrial orchids from seed to mycotrophic plant. Cambridge University Press, New York, USA
Rasmussen HN, Rasmussen FN (1991) Climatic and seasonal regulation of seed plant establishment in Dactylorhiza majalis inferred from symbiotic experiments in vitro. Lindleyana 6:221–227
Rasmussen HN, Andersen TF, Johansen B (1990) Temperature sensitivity of in vitro germination and seedling development of Dactylorhiza majalis (Orchidaceae) with and without a mycorrhizal fungus. Plant Cell Environ 13:171–177
Rasmussen HN, Johansen B, Andersen TF (1991) Symbiotic in vitro culture of immature embryos and seeds of Listera obovata. Lindleyana 6:134–139
Sauleda RP (1976) Harvesting times of orchid seed capsules for the green pod culture process. Am Orchid Soc Bull 45:305–309
Smith SE (1973) Asymbiotic germination of orchid seeds on carbohydrates of fungal origin. New Phytol 72:497–499
Smreciu EA, Currah RS (1989) Symbiotic germination of seeds of terrestrial orchids of North America and Europe. Lindleyana 1:6–15
Stoutamire WP (1963) Terrestrial orchid seedlings. Aust Plants 2:119–122
Stoutamire WP (1964) Seeds and seedlings of native orchids. Mich Bot 3:107–109
Stoutamire WP (1974) Terrestrial orchid seedlings. In: Withner DL (ed) The orchids: scientific studies. Wiley & Sons, New York, USA
Stoutamire WP (1990) Eastern American Cypripedium species and the biology of Cypripedium candidum. In: Sawyers CE (ed) North American native terrestrial orchid propagation and production. Chadds Ford Press, Pennsylvania, USA
Strauss MS, Reisinger DM (1976) Effects of naphthaleneacetic acid on seed germination. Am Orchid Soc Bull 45:722–723
Thompson PA (1974) Orchids from seed: a new basal medium. Orchid Rev 82:179–183
Thompson DI (2003) Conservation of select South African Disa Berg. species (Orchidaceae) through in vitro seed germination. PhD Thesis, University of Natal, Pietermaritzburg
Thompson DI, Edwards TJ, Van Staden J (2001) In vitro germination of several South African summer-rainfall Disa (Orchidaceae) species: is seed testa structure a function of habitat and a determinant of germinability? Syst Geog Plants 71:597–606
Thornhill A, Koopowitz H (1992) Viability of Disa uniflora Berg. (Orchidaceae) seeds under variable storage conditions: is orchid gene banking possible? Biol Conserv 62:21–27
Tomita M, Tomita M (1997) Effects of culture media and cold treatments on germination in asymbiotic culture of Cypripedium marcanthos and C. japonicum. Lindleyana 12:208–213
Ueda H, Torikata H (1972) Effects of light and culture medium on adventitious root formation by Cymbidiums in aseptic culture. Am Orchid Soc Bull 41:322–327
Van der Kinderen G (1987) Abscisic acid in terrestrial orchid seeds: a possible impact on their germination. Lindleyana 2:84–87
Van Waes JM (1987) Effect of activated charcoal on in vitro propagation of western European orchids. Acta Hort 212:131–138
Van Waes JM, Debergh PC (1986a) Adaptation of the tetrazolium method for testing the seed viability, and scanning electron microscopy study of some western European orchids. Physiol Plant 66:435–442
Van Waes JM, Debergh PC (1986b) In vitro germination of some western European orchids. Physiol Plant 67:253–261
Victor JE (2002) South Africa. In: Golding JS (ed) Southern African plant red data lists. Southern African Botanical Diversity Network Report 14. SABONET, Pretoria, South Africa
Vogelpoel L (1980) Disa uniflora—its propagation and culture. Am Orchid Soc Bull 49:961–974
Vogelpoel L (1987) New horizons in Disa breeding. The parent species and their culture I. Orchid Rev 95:176–181
Vogelpoel L (1993) A cultural calendar for Disas based on seasonal biorhythms. S Afr Orchid J 24:37–40
Vujanovic V, St-Arnaud M, Barabé D, Thibeault G (2000) Viability testing of orchid seed and the promotion of colouration and germination. Ann Bot 86:79–86
Warcup JH (1973) Symbiotic germination of some Australian terrestrial orchids. New Phytol 72:387–392
Waterman PG, Mole S (1994) Methods in ecology: analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford, UK
Werckmeister P (1971) Light induction of geotropism, and the control of proliferation and growth of Cymbidium in tissue culture. Bot Gaz 132:346–350
Withner CL (ed) (1959) Orchid physiology. In: The orchids. a scientific survey. Ronald Press, New York, USA
Wodrich K (1997) Growing South African indigenous orchids. AA Balkema, Rotterdam, the Netherlands
Yam TW, Weatherhead MA (1988) Germination and seedling development of some Hong Kong orchids. I. Lindleyana 3:156–160
Yam TW, Ernst R, Arditti J, Nair H, Weatherhead MA (1990) Charcoal in orchid seed germination and tissue culture media: a review. Lindleyana 5:256–265
Yanagawa T, Nagai M, Ogino T, Maeguchi R (1995) Application of disinfectants to orchid seeds, plantlets and media as a means to prevent in vitro contamination. Lindleyana 10:33–36
Yanetti RA (1990) Arethusa bulbosa: germination and culture. In: Sawyers CE (ed) North American native terrestrial orchid propagation and production. Chadds Ford Press, Pennsylvania, USA
Zettler LW, Hofer CJ (1998) Propagation of the little club-spur orchid (Platanthera clavellata) by symbiotic germination and its ecological implications. Environ Exp Bot 39:189–195
Zettler LW, McInnis TM Jr (1992) Propagation of Platanthera intergrilabia (Correll) Luer, an endangered terrestrial orchid, through symbiotic seed germination. Lindleyana 7:154–161
Zettler LW, McInnis TM Jr (1993) Symbiotic seed germination and development of Spiranthes cernua and Goodyera pubescens (Orchidaceae: Spiranthoideae). Lindleyana 8:155–162
Zettler LW, McInnis TM Jr (1994) The effect of white light on the symbiotic seed germination of an endangered orchid, Platanthera integrilabia. Assoc S East Biol Bull 41:129
Acknowledgements
The authors wish to thank the National Research Foundation (NRF) of South Africa and the University of KwaZulu-Natal Research Fund for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, D.I., Edwards, T.J. & van Staden, J. Evaluating asymbiotic seed culture methods and establishing Disa (Orchidaceae) germinability in vitro: relationships, requirements and first-time reports. Plant Growth Regul 49, 269–284 (2006). https://doi.org/10.1007/s10725-006-9137-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-006-9137-z