Abstract
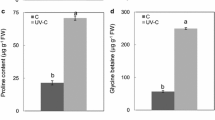
In the southeast of the Qinghai-Tibetan Plateau of China, Mono Maple is a common species in reforestation processes. The paper mainly investigated the changes in morphological, photosynthetic and physiological responses of Mono Maple seedlings to UV-B radiation, nitrogen supply and their combination. The experimental design included two levels of UV-B treatments (ambient UV-B, 11.02 KJ m−2 day−1; enhanced UV-B, 14.33 KJ m−2 day−1) and two nitrogen levels (0; 20 g N m−2 a−1)—to determine whether the adverse effects of UV-B on plants are eased by nitrogen supply. Enhanced UV-B caused a marked decline in growth parameters, net photosynthetic rate, and photosynthetic pigments, whereas it induced an increase in reaction oxygen species (hydrogen peroxide accumulation and the rate of superoxide radical production) and malondialdehyde content. Enhance UV-B also induced an increase in antioxidant compounds of Mono Maple, such as UV-B absorbing compounds, proline content, and activities of antioxidant enzymes (peroxidase, superoxide dimutase and catalase). On the other hand, nitrogen supply caused an increase in some growth parameters, net photosynthetic rate, photosynthetic pigments and antioxidant compounds (peroxidase, proline content and UV-B absorbing compounds), and reduced the content of reaction oxygen species (H2O2 accumulation, the rate of O − 2 production) and malondialdehyde content under ambient UV-B. However, under enhanced UV-B, nitrogen supply inhibited some growth parameters, and increased H2O2 accumulation, the rate of O − 2 production and MDA content, though proline content, UV-B absorbing compounds and activities of POD and SOD increased. These results implied that enhanced UV-B brought harmful effects on Mono Maple seedlings and nitrogen supply made plants more sensitive to enhanced UV-B, though increased some antioxidant activity.




Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione redutase
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- O −2 :
-
Superoxide radical
- POD:
-
Peroxidase
- ROS:
-
Reaction oxygen species
- SOD:
-
Superoxide dimutase
References
Ahmad I, Hellebust JA (1988) The relationship between inorganic nitrogen metabolism and proline accumulation in osmoregulatory responses of two euryhaline microalgae. Plant Physiol 88:348–354
Allen DJ, Nogués S, Baker NR (1998) Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot 49:1775–1788
Antonelli F, Grifoni D, Sabatini F, Zipoli G (1997) Morphological and physiological responses of bean plants to supplemental UV radiation in a Mediterranean climate. Plant Ecol 128:127–136
Bates LS, Waldren RP, Teare IK (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Becana M, Aparicio-Tejo P, Irigoyen JJ, Sánchez-Díaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 82:1169–1171
Binkley D, Son Y, Valentine WD (2000) Do forests receive occult inputs of nitrogen? Ecosystems 3:321–331
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manage 196:43–56
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caldwell MM (1971) Solar ultraviolet radiation and the growth and development of higher plants In: Giese AC (ed) Phytophysiology Academic Press, New York, pp 131–177
Casati P, Lara MV, Andreo CS (2001) Regulation of enzymes involved in C4 photosynthesis and the antioxidant metabolism by UV-B radiation in Egeria densa, a submersed aquatic species. Photosynthesis Res 71:251–264
Correia CM, Moutinho-Pereira JM, Coutinho JF, Björn LO, Torres-Pereira JMG (2005) Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: a Mediterranean field study. Eur J Agron 22:337–347
Costa H, Gallego SM, Tomaro ML (2002) Effects of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci 162:939–945
Day TA, Neale PJ (2002) Effects of UV-B radiation on terrestrial and aquatic primary producers. Ann Rev Ecol Syst 33:371–396
De La Rose TM, Aphalo PJ, Lehto T (2003) Effects of ultraviolet-B radiation on growth, mycorrhizas and mineral nutrition of silver birch (Betula pendula Roth) seedlings grown in low-nutrient conditions. Global Change Biol 9:65–73
De La Rose TM, Julkunen-Tiitto R, Lehto T, Aphalo PJ (2001) Secondary metabolites and nutrient concentrations in silver birch seedlings under five levels of daily UV-B exposure and two relative nutrient addition rates. New Phytologist 150:121–131
Deckmyn G, Impens I (1997) Combined effects of enhanced UV-B radiation and nitrogen deficiency on the growth, composition and photosynthesis of rye (Secale cereale). Plant Ecol 128:235–240
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmo-regulation in plants. Plant J 4:215–223
Du YJ, Jin YH (2000) Effect of far ultraviolet radiation on lipid peroxidation and inherent protection system in seedlings of Taxus cuspidata, Chin. J Appl Ecol 11:660–664 (in Chinese)
Ekmekci Y, Terzioglu S (2005) Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic Biochem Physiol 83:69–81
Executive summary (2003) Environmental effects of ozone depletion and its interactions with climate change: 2002 assessment. Photochem Photobiol Sci 2:1–4
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. J Anne Rev Plant Physiol 33:317–345
Götz T, Windhövel U, Böger P, Sandmann G (1999) Protection of photosynthesis against ultraviolet-B radiation by carotenoids in transformants of the Cyanobacterium Synechococcus PCC7942. Plant Physiol 120:599–604
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plant. Plant Growth Regul 21:79–102
Hunt JE, McNeil DL (1998) Nitrogen status affects UV-B sensitivity of cucumber. Aust J Plant Physiol 25:79–86
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Ke D, Wang A, Sun G, Dong L (2002) The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot Sin 44:551–556
Kulandaivelu G, Margatham S, Nedunchezhian N (1989) On the possible control of ultraviolet-B induced response in growth and photosynthetic activities in higher plants. Physiol Plant 76:398–404
Lavola A, Aphalo PJ, Lahti M, Julkunen-Tiitto R (2003) Nutrient availability and the effect of increasing UV-B radiation on secondary plant compounds in Scots pine. Environ Exp Bot 49:49–60
Levizou E, Manetas Y (2001) Combined effects of enhanced UV-B radiation and additional nutrients on growth of two Mediterranean plant species. Plant Ecol 154:181–186
Li DJ, Mo JM, Fang YT, Pen SL, Gundersen P (2003) Impact of nitrogen deposition on forest plants. Acta Ecologica Sinica 23:1891–1900 (in Chinese)
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mackerness SAH, Jordan BRand Thomas B (1999) Reactive oxygen species in the regulation of photosynthetic genes by ultraviolet-B radiation (UV-B: 280–320 nm) in green and etiolated buds of pea (Pisum sativum L.). J Photochem Photobiol B: Biol 48:180–188
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7:402–415
Nakaji T, Fukami M, Dokiya Y, Izuta T (2001) Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus desiflora seedlings. Trees 15:453–461
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Pinto ME, Casati P, Hsu TP, Ku MS, Edwards GE (1999) Effects of UV-B radiation on growth, photosynthesis, UV-B absorbing compounds and NADP-malic enzyme in bean (Phaseolus vulgaris L.) grown under different nitrogen conditions. J Photochem Photobiol B 48:200–209
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Pruvot G, Masimino J, Peltier G, Rey P (1996) Effects of low temperature, high salinity and exogenous ABA on the synthesis of two chloroplastic drought-induced proteins in Solanum tuberosum. Plant Physiol 97:123–131
Ramalho JC, Campos PS, Teixeria M, Nunes MA (1998) Nitrogen dependent changes in antioxidant system and in fatty acid composition of chloroplast membranes from Coffea Arabica L. plants submitted to high irradiance. Plant Sci 135:115–124
Sánchez E, Ruiz JM, Romero L (2002) Proline metabolism in response to nitrogen toxicity in fruit of French bean plants. Sci Hortic 93:225–233
Santos I, Fidalgo F, Almeida JM (2004) Biochemical and ultrastructural changes in leaves of potato plants grown under supplementary UV-B radiation. Plant Sci 167:925–935
Saradhi PP, Alia SA, Prasad KV (1995) Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Commun 209:1–5
Singh A (1996) Growth, physiological, and biochemical responses of three tropical legumes to enhanced UV-B radiation. Can J Bot 74:135–139
Strid A, Chow WS, Anderson JM (1994) UV-B damage and protection at the molecular level in plants. Photosynth Res 39:475–489
Sullivan JH, Teramura AH, Ziska LH (1992) Variation in UV-B sensitivity in plants from a 3000 m elevational gradient in Hawaii. Am J Bot 79:737–743
Tosserams M, Smet J, Magendans E, Rozema J (2001) Nutrient availability influences UV-B sensitivity of Plantago lanceolata. Plant Ecol 154:159–168
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in sea. How can it occur? Biogeochemistry 13:87–115
Xiao K, Zhang RX, Qian WP (1998) The physiological mechanism of senescence and photosynthetic function decline of flag leaf in wheat regulated by nitrogen nutrition. Plant Nutr Fertil Sci 4: 371–378 (in Chinese)
Xu XL, Ou YH, Pei ZY, Zhou CP (2003) Fate of N15 labeled nitrate and ammonium salts added to an alpine meadow in the Qinghai-Xizang Plateau. Acta Bot Sin 45:276–281 (in Chinese)
Yang YQ, Yao YA, Xu G, Li CY (2005) Growth and physiological responses to drought and elevated ultraviolet-B in two contrasting populations of Hippophae Rhamnoides. Physiol Plant 124:431–440
Yu J, Tang XX, Zhang PY, Tian JY, Cai HJ (2004) Effects of CO2 enrichment on photosynthesis, lipid peroxidation and activities of antioxidative enzymes of platymonas subcordiformis subjected to UV-B radiation stress. Acta Botanica Sinica 46:682–690 (in Chinese)
Acknowledgements
During this work the senior author was supported by the National Natural Science Foundation of China (No. 30530630), the Talent Plan of the Chinese Academy of Sciences and “Knowledge Innovation Engineering” of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, X., Liu, Q. Changes in morphological, photosynthetic and physiological responses of Mono Maple seedlings to enhanced UV-B and to nitrogen addition. Plant Growth Regul 50, 165–177 (2006). https://doi.org/10.1007/s10725-006-9116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-006-9116-4