Abstract
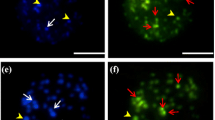
Triple staining with the fluorochromes chromomycin A3, distamycin A and 4′-6-diamidino-2-phenylindole (CMA/DA/DAPI) was applied to somatic metaphases and interphase nuclei of 11 taxa of wild chili peppers (Capsicum), with 2n = 2x = 24 (C. annuum var. glabriusculum, C. cardenasii, C. chacoense, C. flexuosum, C. galapagoense, C. eximium, C. praetermissum and C. tovarii) and 2n = 2x = 26 (C. recurvatum, C. rhomboideum and C. villosum) to analyse heterochromatin type, amount and distribution in wild members of this genus. Heterochromatic banding patterns allowed the identification of all the taxa examined and contributed to their taxonomic grouping. GC-rich heterochromatin (CMA+/DAPI−) was typical in all taxa; only C. praetermissum possessed also AT-rich (CMA−/DAPI+) and mixed GC- and AT-rich (CMA+/DAPI+) bands. Heterochromatin amount (expressed as % of karyotype length) ranged between 1.72 (C. chacoense) and 16.82 (C. flexuosum) and was positively correlated with karyotype length in most of the taxa examined. Heterochromatin located mainly at terminal position of chromosomes but intercalary position prevailed in C. flexuosum. Nucleolus organizer regions (NOR)-associated GC-rich heterochromatin was exclusively terminal and included the distal macrosatellite and a small portion on the corresponding arm. In all the taxa analysed, an equilocal heterochromatin distribution in non-homologous chromosomes of karyotype was observed, suggesting concerted evolution of heterochromatin dispersion in Capsicum.






Similar content being viewed by others
References
Battaglia E (1955) Chromosome morphology and terminology. Caryologia 8:179–187
Bennett MD (1982) The spatial distribution of chromosomes. Kew chromosome conference II. Proceedings of the second chromosome conference, Royal Botanical Gardens, Kew, England
Brandes A, Röder MS, Ganal MW (1995) Barley telomeres are associated with different types of satellite DNA sequences. Chromosome Res 3:315–320
Buso GSC, Pedrosa de Souza Arnaral Z, Bianchetti LB, Ferreira ME (2002) Analise de seqüências de DNA cloroplástico de especies do gênero Capsicum. Boletim de Pesquisa e Desenvolvimento 37 (Embrapa)
Dover GA, Flavell RB (1984) Molecular co-evolution: DNA divergence and the maintenance of function. Cell 38:622–623
Grabiele M (2010) Caracterización citogenética y molecular de secuencias repetidas en el genoma de ajíes (Capsicum—Solanaceae). Doctoral thesis. Instituto Multidisciplinario de Biología Vegetal (IMBIV-CONICET), Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba
Greilhuber J, Loidl J (1983) On regularities of C-banding patterns and their possible causes. In: Brandham PE, Bennett MD (eds) Kew chromosome conference II. Allen & Unwin, London, p 344
Guerra M (2000) Patterns of heterochromatin distribution in plant chromosomes. Genet Mol Biol 23:1029–1041
Guzmán FA, Dean E, Bohs L (2009) Hot or not so hot: phylogenetic relationships in Capsicum and Lycianthes (Solanaceae). Poster presented at the Botany and Mycology 2009 Meeting, Snowbird, Utah. http://2009.botanyconference.org/engine/search/index.php?func=detail&aid=158. Originally with a strict consensus tree, under http://www.biology.utah.edu/bohs/PDFs/Poster-LycCap.jfg
Ibiza VP, Blanca J, Cañizares J, Nuez F (2011) Taxonomy and genetic diversity of domesticated Capsicum species in the Andean region. Genet Resour Crop Evol. doi:10.1007/s10722-011-9744-z
Ince AG, Karaca M, Onus N (2010) Genetic relationships within and between Capsicum species. Biochem Genet 48:83–95
Kenton A (1991) Heterochromatin accumulation, disposition and diversity in Gibasis karwinskyana (Commelinaceae). Chromosoma 100:467–478
Kwon JK, Kim BD (2009) Localization of 5S and 25S rRNA genes on somatic and meiotic chromosomes in Capsicum species of chili pepper. Mol Cells 27(2):205–209. doi:10.1007/s10059-009-0025-z
Lefebvre V, Goffinet B, Chauvet JC, Caromel B, Signoret P, Brand R, Palloix A (2001) Evaluation of genetic distances between pepper inbred lines for cultivar protection purposes: comparison of AFLP, RAPD and phenotypic data. Theor Appl Genet 102:741–750
Levan A, Fredga KL, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Moscone EA (1990) Chromosome studies on Capsicum (Solanaceae) I. Karyotype analysis in C. chacoense. Brittonia 42:147–154
Moscone EA (1993) Estudios cromosómicos en Capsicum (Solanaceae) II. Análisis cariotípico de C. parvifolium y C. annuum var. annuum. Kurtziana 22:9–18
Moscone EA (1999) Análisis cariotípico en C. baccatum var. umbilicatum (Solanaceae) mediante bandeos AgNOR y de fluorescencia. Kurtziana 27:225–232
Moscone EA, Lambrou M, Hunziker AT, Ehrendorfer F (1993) Giemsa C-banded karyotypes in Capsicum (Solanaceae). Plant Syst Evol 186:213–229
Moscone EA, Loidl J, Ehrendorfer F, Hunziker AT (1995) Analysis of active nucleolus organizing regions in Capsicum (Solanaceae) by silver staining. Am J Bot 82:276–287
Moscone EA, Lambrou M, Ehrendorfer F (1996) Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). Plant Syst Evol 202:37–63
Moscone EA, Baranyi M, Ebert I, Greilhuber J, Ehrendorfer F, Hunziker AT (2003) Analysis of nuclear DNA content in Capsicum (Solanaceae) by flow cytometry and Feulgen densitometry. Ann Bot 92:21–29
Moscone EA, Scaldaferro MA, Grabiele M, Cecchini NM, Sánchez García Y, Jarret R, Daviña JR, Ducasse DA, Barboza GE, Ehrendofer F (2007) The evolution of chili peppers (Capsicum—Solanaceae): a cytogenetic perspective. Acta Hortic 745:137–169
Paran I, Aftergoot E, Shifriss C (1998) Variation in C. annuum revealed by RAPD and AFLP markers. Euphytica 99:167–173
Park Y-K, Kim B-D, Kim B-S, Armstrong KC, Kim N-S (1999) Karyotyping of the chromosomes and physical mapping of the 5S rRNA and 18S–26S rRNA gene families in five different species in Capsicum. Genes Genet Syst 74:149–157
Park Y-K, Park K-C, Park C-H, Kim N-S (2000) Chromosomal localization and sequence variation of 5S rRNA gene in five Capsicum species. Mol Cells 10:18–24
Perry DR, Zarrillo S, Holst I, Pearsall DM, Piperno DR, Berman MJ, Cooke RG, Rademaker K, Ranere AJ, Raymond JS, Sandweiss DH, Scaramelli F, Tarble K, Zeidler JA (2007) Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 315:986–988
Pickersgill B (1971) Relationships between weedy and cultivated forms in some species of chili peppers (genus Capsicum). Evolution 25:683–691
Pickersgill B (1991) Cytogenetics and evolution of Capsicum L. In: Tsuchiya T, Gupta PK (eds) Chromosome engineering in plants: genetics, breeding, evolution, part B. Elsevier, Amsterdam, The Netherlands, pp 139–160
Pozzobon MT, Schifino-Wittmann MT, Bianchetti LB (2006) Chromosome numbers in wild and semidomesticated Brazilian Capsicum L. (Solanaceae) species: do x = 12 and x = 13 represent two evolutionary lines? Bot J Linn Soc 151:259–269
Prince JP, Lackney VK, Angeles C, Blauth JR, Kyle MM (1995) A survey of DNA polymorphism within the genus Capsicum and the fingerprinting of pepper cultivars. Genome 38:224–231
Rodriguez JM, Berke T, Engle L, Nienhuis J (1999) Variation among and within Capsicum species revealed by RAPD markers. Theor Appl Genet 99:147–156
Scaldaferro MA, Seijo JG, Acosta MC, Barboza GE, Ducasse DA, Moscone EA (2006) Genomic characterization of the germplasm in peppers (Capsicum—Solanaceae) by fluorescent in situ hybridization. Plant Sci (Sofia) 43:291–297
Schwarzacher T, Mayr B, Schweizer D (1984) Heterochromatin and NOR behaviour at male pachytene of Sus scrofa domestica. Chromosoma 91:12–19
Schweizer D (1979) Fluorescent chromosome banding in plants: applications, mechanisms, and implications for chromosome structure. In: Davies DR, Hopwood DA (eds), The plant genome (Proceedings of the fourth John Innes Symposium, p 61–72). John Innes Charity, Norwich, UK, pp 171–183, (1980)
Schweizer D, Ambros PF (1994) Chromosome banding stain combinations for specific regions. In: Gosden JR (ed) Chromosome analysis protocols. Methods in molecular biology, Vol. 29. Humana Press, Totowa, USA, pp 97–112
Schweizer D, Loidl J (1987) A model for heterochromatin dispersion and the evolution of C-band patterns. In: Stahl A, Luciani JM, Vagner-Capodano AM (eds) Chromosomes today Vol. 9. Allen & Unwin, London, pp 61–74
Tong N, Bosland PW (1999) Capsicum tovarii, a new member of the C. baccatum complex. Euphytica 109:71–77
Tong N, Bosland PW (2003) Observations on interspecific compatibilty and meiotic chromosome behavior of C. buforum and C. lanceolatum. Genet Resour Crop Evol 50:193–199
Votava EJ, Baral JB, Bosland PW (2005) Genetic diversity of chile (C. annuum var. annuum L.) landraces from northern New Mexico, Colorado and Mexico. Econ Bot 59:8–17
Walsh BM, Hoot SB (2001) Phylogenetic relationships of Capsicum (Solanaceae) using DNA sequences from two noncoding regions: the chloroplast a@B-rbcL spacer region and nuclear waxy introns. Int J Plant Sci 162:1409–1418
Acknowledgments
This work was made possible by doctoral scholarship granted by Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba (SECyT-UNC), and is part of the doctoral thesis of Marisel Scaldaferro.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scaldaferro, M.A., Grabiele, M. & Moscone, E.A. Heterochromatin type, amount and distribution in wild species of chili peppers (Capsicum, Solanaceae). Genet Resour Crop Evol 60, 693–709 (2013). https://doi.org/10.1007/s10722-012-9867-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-012-9867-x