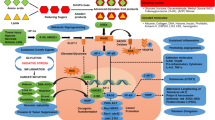
Abstract
Cancer is a complex disease with a 5–10% hereditary base, but nutrition, lifestyle, and the environment we are exposed to influence 90–95% of cancers. Due to rapid westernization, the diet we consume is rich in advanced glycation end products (AGEs). AGEs are the heterogeneous group of compounds formed by non-enzymatic reactions between reducing sugars and amino groups of proteins, lipids, and nucleic acids. Its implication is confirmed in many chronic conditions such as diabetes, renal, cardiovascular diseases, and aging however its role in cancer development has been understudied. Cancer cells are continuously exposed to AGEs due to their increased production, owing to its high metabolic rate and aerobic glycolysis. AGEs accumulation led to glycative stress which in turn stimulates oxidative stress and inflammation, through its receptor known as receptor for advanced glycation end products (RAGE). RAGE mediates crosstalk between the tumour cells and its microenvironment components to induce hypoxia, mitochondrial dysfunction, endoplasmic reticulum stress, autophagy, epigenetic modification, and cancer stemness. This emphasizes AGEs as an essential driving factor in different aspects of cancer development, but the exact molecular mechanism has to be explored. Thus, this review gives an insight into the pathological role of AGEs at the bio-molecular level in the tumourigenesis and progression of cancer in terms of the tumour microenvironment, invasion, and metastasis. Further, the compiled clinical data relating to the AGE-RAGE axis associated with different cancers and its potential inhibitors have been discussed.




Similar content being viewed by others
Abbreviations
- ADAM 10:
-
A disintegrin and metalloproteinase10
- AGEs:
-
Advanced glycation end products
- ALES:
-
Advanced lipoxidation end products
- AP-1:
-
Activator protein 1
- ATF6:
-
Activating transcription factor 6
- Bcl-2:
-
B-cell lymphoma-2
- Bcl-xL:
-
B-cell lymphoma-extra-large
- CEL:
-
Carboxyethyllysine
- ChREBP:
-
Carbohydrate responsive element binding protein
- CML:
-
Carboxymethyllysine
- cRAGE:
-
Cleaved RAGE
- ECM:
-
Extracellular matrix
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated protein kinases
- esRAGE:
-
:Endogenous secretory RAGE
- FAK:
-
Focal adhesion kinase
- GLO-I:
-
Glyoxalase-I
- GLUT1:
-
Glucose transporters 1
- GSK-3β :
-
Glycogen synthase kinase 3 beta
- HIF-1α:
-
Hypoxia inducible factor-1
- HMGB1:
-
High mobility group box 1 protein
- HRE:
-
Hypoxia-response elements
- ICAM-1:
-
Intercellular cell adhesion molecule 1
- IL-1/6:
-
Interleukin 1/6
- IRE1α:
-
Inositol-requiring enzyme 1α
- JAK/ STAT:
-
Janus kinase/signal transducers and activators of transcription
- JNK:
-
C-Jun N-terminal kinases
- MAMs:
-
Mitochondrial associated molecular membrane
- MAPK:
-
Mitogen-activated protein kinases
- MG:
-
Methylglyoxal
- MMP:
-
Matrix metalloproteinases
- MSR-1:
-
Macrophage scavenger receptor-1
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor kappa B
- NOX:
-
NADPH oxidase
- Nrf-2:
-
Nuclear factor erythroid 2–related factor 2
- PARP:
-
Poly-ADP-ribose polymerase
- PDGF:
-
Platelet-derived growth factor
- PERK:
-
Pancreatic ER kinase-like ER kinase
- RAGE:
-
Receptor for AGEs
- ROS:
-
Reactive oxygen species
- sRAGE:
-
Soluble RAGE
- TGF-β :
-
Transforming growth factor-β
- TNF-α:
-
Tumour necrosis factor- α
- VCAM-1:
-
Vascular cell adhesion molecule
- VEGF:
-
Vascular endothelial growth factor
References
Monnier, V.M.: Nonenzymatic Glycosylation, the Maillard Reaction and the Aging Process. J. Gerontol. 45, B105–B111 (1990). https://doi.org/10.1093/GERONJ/45.4.B105
Luevano-Contreras, C., Chapman-Novakofski, K.: Dietary advanced glycation end products and aging. Nutrients 2, 1247–1265 (2010). https://doi.org/10.3390/nu2121247
Warburg, O.: The metabolism of carcinoma cells 1. The Journal of Cancer Research. 9, 148–163 (1925). https://doi.org/10.1158/jcr.1925.148
Njoroge, F.G., Monnier, V.M.: The chemistry of the Maillard reaction under physiological conditions: a review. Prog. Clin. Biol. Res. 304, 85–107 (1989)
Goldberg, T., Cai, W., Peppa, M., Dardaine, V., Baliga, B.S., Uribarri, J., Vlassara, H.: Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 104, 1287–1291 (2004). https://doi.org/10.1016/j.jada.2004.05.214
Glomb, M.A., Monnier, V.M.: Mechanism of Protein Modification by Glyoxal and Glycolaldehyde, Reactive Intermediates of the Maillard Reaction (∗). J. Biol. Chem. 270, 10017–10026 (1995). https://doi.org/10.1074/JBC.270.17.10017
Phillips, S.A., Thornalley, P.J.: The formation of methylglyoxal from triose phosphates: Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 212, 101–105 (1993). https://doi.org/10.1111/J.1432-1033.1993.TB17638.X
Vistoli, G., de Maddis, D., Cipak, A., Zarkovic, N., Carini, M., Aldini, G.: Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radical Res. 47, 3–27 (2013). https://doi.org/10.3109/10715762.2013.815348
Srikrishna, G., Nayak, J., Weigle, B., Temme, A., Foell, D., Hazelwood, L., Olsson, A., Volkmann, N., Hanein, D., Freeze, H.H.: Carboxylated N-glycans on RAGE promote S100A12 binding and signaling. J. Cell. Biochem. 110, 645–659 (2010). https://doi.org/10.1002/jcb.22575
Dattilo, B.M., Fritz, G., Leclerc, E., vander Kooi, C.W., Heizmann, C.W., Chazin, W.J.: The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units. Biochemistry 46, 6957–6970 (2007). https://doi.org/10.1021/bi7003735
Koch, M., Chitayat, S., Dattilo, B.M., Schiefner, A., Diez, J., Chazin, W.J., Fritz, G.: Structural Basis for Ligand Recognition and Activation of RAGE. Structure. 18, 1342–1352 (2010). https://doi.org/10.1016/j.str.2010.05.017
Indurthi, V.S.K., Jensen, J.L., Leclerc, E., Sinha, S., Colbert, C.L., Vetter, S.W.: The TRP triad within the V-domain of the receptor for advanced glycation end products modulates folding, stability and ligand binding. Biosci. Rep. 40, (2020). https://doi.org/10.1042/BSR20193360
Moysa, A., Hammerschmid, D., Szczepanowski, R.H., Sobott, F., Dadlez, M.: Enhanced oligomerization of full-length RAGE by synergy of the interaction of its domains. Sci. Rep. 9, 1–15 (2019). https://doi.org/10.1038/s41598-019-56993-9
Yonekura, H., Yamamoto, Y., Sakurai, S., Petrova, R.G., Abedin, M.J., Li, H., Yasui, K., Takeuchi, M., Makita, Z., Takasawa, S., Okamoto, H., Watanabe, T., Yamamoto, H.: Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. J. Biol. Chem. 370, 1097–1109 (2003). https://doi.org/10.1042/BJ20021371
Hanford, L.E., Enghild, J.J., Valnickova, Z., Petersen, S. v., Schaefer, L.M., Schaefer, T.M., Reinhart, T.A., Oury, T.D.: Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J. Biol. Chem. 279, 50019–50024 (2004). https://doi.org/10.1074/jbc.M409782200
Basta, G., Leonardis, D., Mallamaci, F., Cutrupi, S., Pizzini, P., Gaetano, L., Tripepi, R., Tripepi, G., de Caterina, R., Zoccali, C.: Circulating soluble receptor of advanced glycation end product inversely correlates with atherosclerosis in patients with chronic kidney disease. Kidney Int. 77, 225–231 (2010). https://doi.org/10.1038/ki.2009.419
Tesařová, P., Kalousová, M., Jáchymová, M., Mestek, O., Petruzelka, L., Zima, T.: Receptor for advanced glycation end products (RAGE) - Soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Invest. 25, 720–725 (2007). https://doi.org/10.1080/07357900701560521
Jing, R., Cui, M., Wang, J., Wang, H.: Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): A new biomarker for lung cancer. Neoplasma 57, 55–61 (2010). https://doi.org/10.4149/neo_2010_01_055
Krechler, T., Jáchymová, M., Mestek, O., Žák, A., Zima, T., Kalousová, M.: Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clin. Biochem. 43, 882–886 (2010). https://doi.org/10.1016/j.clinbiochem.2010.04.004
Jiao, L., Taylor, P.R., Weinstein, S.J., Graubard, B.I., Virtamo, J., Albanes, D., Stolzenberg-Solomon, R.Z.: Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 20, 1430–1438 (2011). https://doi.org/10.1158/1055-9965.EPI-11-0066
Zhou, X., Lin, N., Zhang, M., Wang, X., An, Y., Su, Q., Du, P., Li, B., Chen, H.: Circulating soluble receptor for advanced glycation end products and other factors in type 2 diabetes patients with colorectal cancer. BMC Endocr. Disord. 20, 1–7 (2020). https://doi.org/10.1186/s12902-020-00647-9
Sullivan, L.B., Gui, D.Y., van der Heiden, M.G.: Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer 16, 680–693 (2016). https://doi.org/10.1038/nrc.2016.85
Hakimi, A.A., Reznik, E., Lee, C.H., Creighton, C.J., Brannon, A.R., Luna, A., Aksoy, B.A., Liu, E.M., Shen, R., Lee, W., Chen, Y., Stirdivant, S.M., Russo, P., Chen, Y.B., Tickoo, S.K., Reuter, V.E., Cheng, E.H., Sander, C., Hsieh, J.J.: An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 29, 104–116 (2016). https://doi.org/10.1016/j.ccell.2015.12.004
Kim, D., Fiske, B.P., Birsoy, K., Freinkman, E., Kami, K., Possemato, R.L., Chudnovsky, Y., Pacold, M.E., Chen, W.W., Cantor, J.R., Shelton, L.M., Gui, D.Y., Kwon, M., Ramkissoon, S.H., Ligon, K.L., Kang, S.W., Snuderl, M., vander Heiden, M.G., Sabatini, D.M.: SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520, 363–367 (2015). https://doi.org/10.1038/nature14363
Rabbani, N., Thornalley, P.J.: Dicarbonyl proteome and genome damage in metabolic and vascular disease. https://pubmed.ncbi.nlm.nih.gov/24646255/. (2014)
He, R.-Q., Yang, M.-D., Zheng, X., Zhou, J.-X.: Isolation and some properties of glycated D-glyceraldehyde-3-phosphate dehydrogenase from rabbit muscle. (1995)
Morgan, P.E., Dean, R.T., Davies, M.J.: Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch. Biochem. Biophys. 403, 259–269 (2002). https://doi.org/10.1016/S0003-9861(02)00222-9
Bellier, J., Nokin, M.J., Lardé, E., Karoyan, P., Peulen, O., Castronovo, V., Bellahcène, A.: Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. https://pubmed.ncbi.nlm.nih.gov/30664892/. (2019)
Moraru, A., Wiederstein, J., Pfaff, D., Fleming, T., Miller, A.K., Nawroth, P., Teleman, A.A.: Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell Metab. 27, 926-934.e8 (2018). https://doi.org/10.1016/j.cmet.2018.02.003
Bian, Y., Yu, Y., Wang, S., Li, L.: Up-regulation of fatty acid synthase induced by EGFR/ERK activation promotes tumor growth in pancreatic cancer. Biochem. Biophys. Res. Commun. 463, 612–617 (2015). https://doi.org/10.1016/J.BBRC.2015.05.108
van Heijst, J.W.J., Niessen, H.W.M., Musters, R.J., van Hinsbergh, V.W.M., Hoekman, K., Schalkwijk, C.G.: Argpyrimidine-modified Heat Shock Protein 27 in human non-small cell lung cancer: A possible mechanism for evasion of apoptosis. Cancer Lett. 241, 309–319 (2006). https://doi.org/10.1016/J.CANLET.2005.10.042
GLO1 overexpression in human malignant melanoma: Wb, B., Cm, C., K, U., AS, B., GT, W. Melanoma Res. 20, 85–96 (2010). https://doi.org/10.1097/CMR.0B013E3283364903
Oya-Ito Tomoko, T., Naito, Y., Takagi, T., Handa, O., Matsui, H., Yamada, M., Shima, K., Yoshikawa, T.: Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochimica et Biophysica Acta - Molecular Basis of Disease. 1812, 769–781 (2011). https://doi.org/10.1016/j.bbadis.2011.03.017
Nokin, M.J., Durieux, F., Peixoto, P., Chiavarina, B., Peulen, O., Blomme, A., Turtoi, A., Costanza, B., Smargiasso, N., Baiwir, D., Scheijen, J.L., Schalkwijk, C.G., Leenders, J., de Tullio, P., Bianchi, E., Thiry, M., Uchida, K., Spiegel, D.A., Cochrane, J.R., Hutton, C.A., de Pauw, E., Delvenne, P., Belpomme, D., Castronovo, V., Bellahcène, A.: Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP- mediated tumor growth and metastasis. eLife. 5, (2016). https://doi.org/10.7554/eLife.19375
Sun, F., Suttapitugsakul, S., Xiao, H., Wu, R.: Comprehensive Analysis of Protein Glycation Reveals Its Potential Impacts on Protein Degradation and Gene Expression in Human Cells. J. Am. Soc. Mass Spectrom. 30, 2480–2490 (2019). https://doi.org/10.1007/s13361-019-02197-4
Shimomoto, T., Luo, Y., Ohmori, H., Chihara, Y., Fujii, K., Sasahira, T., Denda, A., Kuniyasu, H.: Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J. Gastroenterol. 47, 1073–1083 (2012). https://doi.org/10.1007/s00535-012-0572-5
Thimmulappa, R.K., Mai, K.H., Srisuma, S., Kensler, T.W., Yamamoto, M., Biswal, S.: Identification of Nrf2-regulated Genes Induced by the Chemopreventive Agent Sulforaphane by Oligonucleotide Microarray 1. (2002)
Fu, J., Xiong, Z., Huang, C., Li, J., Yang, W., Han, Y., Paiboonrungruan, C., Major, M.B., Chen, K.N., Kang, X., Chen, X.: Hyperactivity of the transcription factor Nrf2 causes metabolic reprogramming in mouse esophagus. J. Biol. Chem. 294, 327–340 (2019). https://doi.org/10.1074/jbc.RA118.005963
Isoe, T., Makino, Y., Mizumoto, K., Sakagami, H., Fujita, Y., Honjo, J., Takiyama, Y., Itoh, H., Haneda, M.: High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. (2010). https://doi.org/10.1038/ki.2010.99
Li, J., Zhao, S.Z., Wang, P.P., Yu, S.P., Zheng, Z., Xu, X.: Calcium mediates high glucose-induced HIF-1α and VEGF expression in cultured rat retinal Müller cells through CaMKII-CREB pathway. Acta Pharmacol. Sin. 33, 1030–1036 (2012). https://doi.org/10.1038/aps.2012.61
Gordan, J.D., Bertout, J.A., Hu, C.J., Diehl, J.A., Simon, M.C.: HIF-2α Promotes Hypoxic Cell Proliferation by Enhancing c-Myc Transcriptional Activity. Cancer Cell 11, 335–347 (2007). https://doi.org/10.1016/j.ccr.2007.02.006
Kim, J., Zeller, K.I., Wang, Y., Jegga, A.G., Aronow, B.J., O’Donnell, K.A., Dang, C.V.: Evaluation of Myc E-Box Phylogenetic Footprints in Glycolytic Genes by Chromatin Immunoprecipitation Assays. Mol. Cell. Biol. 24, 5923–5936 (2004). https://doi.org/10.1128/mcb.24.13.5923-5936.2004
Osthus, R.C., Shim, H., Kim, S., Li, Q., Reddy, R., Mukherjee, M., Xu, Y., Wonsey, D., Lee, L.A., Dang, C.V.: Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 275, 21797–21800 (2000). https://doi.org/10.1074/jbc.C000023200
Gan, L., Xiu, R., Ren, P., Yue, M., Su, H., Guo, G., Xiao, D., Yu, J., Jiang, H., Liu, H., Hu, G., Qing, G.: Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene 35, 3037–3048 (2016). https://doi.org/10.1038/onc.2015.360
Viola, A., Munari, F., Sánchez-Rodríguez, R., Scolaro, T., Castegna, A.: The metabolic signature of macrophage responses. Front. Immunol. 10, 1–16 (2019). https://doi.org/10.3389/fimmu.2019.01462
Li, D., Ma, S., Ellis, E.M.: Nrf2-mediated adaptive response to methyl glyoxal in HepG2 cells involves the induction of AKR7A2. Chem. Biol. Interact. 234, 366–371 (2015). https://doi.org/10.1016/j.cbi.2014.10.019
Zemva, J., Fink, C.A., Fleming, T.H., Schmidt, L., Loft, A., Herzig, S., Knieß, R.A., Mayer, M., Bukau, B., Nawroth, P.P., Tyedmers, J.: Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox Biol. 13, 674–686 (2017). https://doi.org/10.1016/j.redox.2017.08.007
Bollong, M.J., Lee, G., Coukos, J.S., Yun, H., Zambaldo, C., Chang, J.W., Chin, E.N., Ahmad, I., Chatterjee, A.K., Lairson, L.L., Schultz, P.G., Moellering, R.E.: A metabolite-derived protein modification integrates glycolysis with KEAP1–NRF2 signalling. Nature 562, 600–604 (2018). https://doi.org/10.1038/s41586-018-0622-0
Chuah, Y.K., Basir, R., Talib, H., Tie, T.H., Nordin, N.: Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inflamm. 2013, (2013). https://doi.org/10.1155/2013/403460
Lin, L., Park, S., Lakatta, E.G.: RAGE signaling in inflammation and arterial aging. Front. Biosci. 14, 1403–1413 (2009). https://doi.org/10.2741/3315
Sakasai-Sakai, A., Takeuchi, M., Takata, T.: Intracellular toxic advanced glycation end-products promote the production of reactive oxygen species in HEPG2 cells. Int. J. Mol. Sci. 21, 1–14 (2020). https://doi.org/10.3390/ijms21144861
Nedić, O., Rattan, S.I.S., Grune, T., Trougakos, I.P.: Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radical Res. 47, 28–38 (2013). https://doi.org/10.3109/10715762.2013.806798
Rojas, A., Figueroa, H., Morales, E.: Fueling inflammation at tumor microenvironment: The role of multiligand/rage axis. Carcinogenesis 31, 334–341 (2010). https://doi.org/10.1093/carcin/bgp322
Kang, R., Tang, D., Schapiro, N.E., Livesey, K.M., Farkas, A., Loughran, P., Bierhaus, A., Lotze, M.T., Zeh, H.J.: The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 17, 666–676 (2010). https://doi.org/10.1038/cdd.2009.149
Petriv, N., Neubert, L., Vatashchuk, M., Timrott, K., Suo, H., Hochnadel, I., Huber, R., Petzold, C., Hrushchenko, A., Yatsenko, A.S., Shcherbata, H.R., Wedemeyer, H., Lichtinghagen, R., Falfushynska, H., Lushchak, V., Manns, M.P., Bantel, H., Semchyshyn, H., Yevsa, T.: Increase of α-dicarbonyls in liver and receptor for advanced glycation end products on immune cells are linked to nonalcoholic fatty liver disease and liver cancer. OncoImmunology 10, (2021). https://doi.org/10.1080/2162402X.2021.1874159
Rosenstock, P., Bezold, V., Bork, K., Scheffler, J., Horstkorte, R.: Glycation interferes with natural killer cell function. Mech. Ageing Dev. 178, 64–71 (2019). https://doi.org/10.1016/j.mad.2019.01.006
Grivennikov, S.I., Karin, M.: Inflammation and oncogenesis: a vicious connection. Curr. Opin. Genet. Dev. 20, 65–71 (2010). https://doi.org/10.1016/j.gde.2009.11.004
Niu, G., Wright, K.L., Ma, Y., Wright, G.M., Huang, M., Irby, R., Briggs, J., Karras, J., Cress, W.D., Pardoll, D., Jove, R., Chen, J., Yu, H.: Role of Stat3 in regulating p53 expression and function. Mol. Cell. Biol. 25, 7432–7440 (2005). https://doi.org/10.1128/MCB.25.17.7432-7440.2005
Tergaonkar, V., Pando, M., Vafa, O., Wahl, G., Verma, I.: p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell 1, 493–503 (2002). https://doi.org/10.1016/s1535-6108(02)00068-5
Koh, M.Y., Powis, G.: Passing the baton: The HIF switch. http://www.ncbi.nlm.nih.gov/pubmed/22818162. (2012)
Wang, M., Kirk, J.S., Venkataraman, S., Domann, F.E., Zhang, H.J., Schafer, F.Q., Flanagan, S.W., Weydert, C.J., Spitz, D.R., Buettner, G.R., Oberley, L.W.: Manganese superoxide dismutase suppresses hypoxic induction of hypoxia-inducible factor-1α and vascular endothelial growth factor. Oncogene 24, 8154–8166 (2005). https://doi.org/10.1038/sj.onc.1208986
Xu, Y., Toure, F., Qu, W., Lin, L., Song, F., Shen, X., Rosario, R., Garcia, J., Schmidt, A.M., Yan, S.F.: Advanced glycation end product (AGE)-receptor for age (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J. Biol. Chem. 285, 23233–23240 (2010). https://doi.org/10.1074/jbc.M110.117457
Masson, N., Ratcliffe, P.J.: Hypoxia signaling pathways in cancer metabolism: The importance of co-selecting interconnected physiological pathways. (2014)
Fuhrmann, D.C., Brüne, B.: Mitochondrial composition and function under the control of hypoxia. Redox Biol. 12, 208–215 (2017). https://doi.org/10.1016/j.redox.2017.02.012
Patel, S.H., Yue, F., Saw, S.K., Foguth, R., Cannon, J.R., Shannahan, J.H., Kuang, S., Sabbaghi, A., Carroll, C.C.: Advanced Glycation End-Products Suppress Mitochondrial Function and Proliferative Capacity of Achilles Tendon-Derived Fibroblasts. Sci. Rep. 9, 1–17 (2019). https://doi.org/10.1038/s41598-019-49062-8
Bonner, M.R., Shen, M., Liu, C.S., DiVita, M., He, X., Lan, Q.: Mitochondrial DNA content and lung cancer risk in Xuan Wei. China. Lung Cancer. 63, 331–334 (2009). https://doi.org/10.1016/j.lungcan.2008.06.012
Dioufa, N., Kassi, E., Papavassiliou, A.G., Kiaris, H.: Atypical induction of the unfolded protein response by mifepristone. Endocrine 38, 167–173 (2010). https://doi.org/10.1007/s12020-010-9362-0
Healy, S.J.M., Gorman, A.M., Mousavi-Shafaei, P., Gupta, S., Samali, A.: Targeting the endoplasmic reticulum-stress response as an anticancer strategy. http://www.ncbi.nlm.nih.gov/pubmed/19835867. (2009)
Koong, A.C., Chauhan, V., Romero-Ramirez, L.: Targeting XBP-1 as a novel anti-cancer strategy. http://www.ncbi.nlm.nih.gov/pubmed/16861911. (2006)
Hotamisligil, G.S.: Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. http://www.ncbi.nlm.nih.gov/pubmed/20303879. (2010)
Piperi, C., Adamopoulos, C., Dalagiorgou, G., Diamanti-Kandarakis, E., Papavassiliou, A.G.: Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 97, 2231–2242 (2012). https://doi.org/10.1210/jc.2011-3408
Rasheed, Z., Akhtar, N., Haqqi, T.M.: Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology (Oxford) 50, 838–851 (2011). https://doi.org/10.1093/rheumatology/keq380
Logsdon, C., Fuentes, M., Huang, E., Arumugam, T.: RAGE and RAGE Ligands in Cancer. Curr. Mol. Med. 7, 777–789 (2007). https://doi.org/10.2174/156652407783220697
Missiroli, S., Patergnani, S., Caroccia, N., Pedriali, G., Perrone, M., Previati, M., Wieckowski, M.R., Giorgi, C.: Mitochondria-associated membranes (MAMs) and inflammation, (2018)
Murakami, T., Ockinger, J., Yu, J., Byles, V., McColl, A., Hofer, A.M., Horng, T.: Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. U.S.A. 109, 11282–11287 (2012). https://doi.org/10.1073/pnas.1117765109
Fallah, A., Sadeghinia, A., Kahroba, H., Samadi, A., Heidari, H.R., Bradaran, B., Zeinali, S., Molavi, O.: Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. (2019)
Muoio, M.G., Talia, M., Lappano, R., Sims, A.H., Vella, V., Cirillo, F., Manzella, L., Giuliano, M., Maggiolini, M., Belfiore, A., de Francesco, E.M.: Activation of the s100a7/rage pathway by igf-1 contributes to angiogenesis in breast cancer. Cancers 13, 1–19 (2021). https://doi.org/10.3390/cancers13040621
Okamoto, T., Yamagishi, S.I., Inagaki, Y., Amano, S., Koga, K., Abe, R., Takeuchi, M., Ohno, S., Yoshimura, A., Makita, Z.: Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. The FASEB Journal : official publication of the Federation of American Societies for Experimental Biology 16, 1928–1930 (2002). https://doi.org/10.1096/fj.02-0030fje
Duyndam, M.C.A., Hulscher, T.M., Fontijn, D., Pinedo, H.M., Boven, E.: Induction of Vascular Endothelial Growth Factor Expression and Hypoxia-inducible Factor 1α Protein by the Oxidative Stressor Arsenite. J. Biol. Chem. 276, 48066–48076 (2001). https://doi.org/10.1074/jbc.M106282200
Yamagishi, S.I., Amano, S., Inagaki, Y., Okamoto, T., Koga, K., Makita, Z., Sasaki, N., Yamamoto, H., Takeuchi, M.: Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 290, 973–978 (2002). https://doi.org/10.1006/bbrc.2001.6312
Liang, H., Zhong, Y., Zhou, S., Peng, L.: Knockdown of RAGE expression inhibits colorectal cancer cell invasion and suppresses angiogenesis in vitro and in vivo. Cancer Lett. 313, 91–98 (2011). https://doi.org/10.1016/j.canlet.2011.08.028
Mathew, R., Karantza-Wadsworth, V., White, E.: Role of autophagy in cancer. (2007)
Li, W., Saud, S.M., Young, M.R., Chen, G., Hua, B.: Targeting AMPK for cancer prevention and treatment. 6, (2015)
Li, J., Wu, P.W., Zhou, Y., Dai, B., Zhang, P.F., Zhang, Y.H., Liu, Y., Shi, X.L.: Rage induces hepatocellular carcinoma proliferation and sorafenib resistance by modulating autophagy article. Cell Death Dis 9, (2018). https://doi.org/10.1038/s41419-018-0329-z
Verma, N., Manna, S.K.: Advanced glycation end products (AGE) potently induce autophagy through activation of RAF protein kinase and nuclear factor κB (NF-κB). J. Biol. Chem. 291, 1481–1491 (2016). https://doi.org/10.1074/jbc.M115.667576
Z, Z., H, W., L, Z., X, M., J, H., K, H.: Receptor for advanced glycation end product blockade enhances the chemotherapeutic effect of cisplatin in tongue squamous cell carcinoma by reducing autophagy and modulating the WNT pathway. Anti-cancer Drugs 28, 187–196 (2017). https://doi.org/10.1097/CAD.0000000000000451
Swami, P., O’connell, K.A., Thiyagarajan, S., Crawford, A., Patil, P., Radhakrishnan, P., Shin, S., Caffrey, T.C., Grunkemeyer, J., Neville, T., Vetter, S.W., Hollingsworth, M.A., Leclerc, E.: Inhibition of the receptor for advanced glycation end products enhances the cytotoxic effect of gemcitabine in murine pancreatic tumors. Biomolecules 11, (2021). https://doi.org/10.3390/biom11040526
Yuan, X., Wang, B., Yang, L., Zhang, Y.: The role of ROS-induced autophagy in hepatocellular carcinoma. https://pubmed.ncbi.nlm.nih.gov/29544680/. (2018)
Zheng, Q., Omans, N.D., Leicher, R., Osunsade, A., Agustinus, A.S., Finkin-Groner, E., D’Ambrosio, H., Liu, B., Chandarlapaty, S., Liu, S., David, Y.: Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 10, (2019). https://doi.org/10.1038/s41467-019-09192-z
Guedes, S., Vitorino, R., Domingues, M.R.M., Amado, F., Domingues, P.: Glycation and oxidation of histones H2B and H1: In vitro study and characterization by mass spectrometry. Anal. Bioanal. Chem. 399, 3529–3539 (2011). https://doi.org/10.1007/s00216-011-4679-y
Diao, X.: Histone glycation: Linking metabolic perturbation with epigenetic misregulation in cancer. AIMS Genetics. 6, 14–16 (2019). https://doi.org/10.3934/genet.2019.2.14
Brasacchio, D., Okabe, J., Tikellis, C., Balcerczyk, A., George, P., Baker, E.K., Calkin, A.C., Brownlee, M., Cooper, M.E., El-Osta, A.: Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58, 1229–1236 (2009). https://doi.org/10.2337/db08-1666
Sheppard, K.-A., Rose, D.W., Haque, Z.K., Kurokawa, R., McInerney, E., Westin, S., Thanos, D., Rosenfeld, M.G., Glass, C.K., Collins, T.: Transcriptional Activation by NF-κB Requires Multiple Coactivators. Mol. Cell. Biol. 19, 6367–6378 (1999). https://doi.org/10.1128/mcb.19.9.6367
Brings, S., Fleming, T., Freichel, M., Muckenthaler, M.U., Herzig, S., Nawroth, P.P.: Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention, /pmc/articles/PMC5454897/. (2017)
Scumaci, D., Olivo, E., Fiumara, C.V., la Chimia, M., de Angelis, M.T., Mauro, S., Costa, G., Ambrosio, F.A., Alcaro, S., Agosti, V., Costanzo, F.S., Cuda, G.: DJ-1 Proteoforms in Breast Cancer Cells: The Escape of Metabolic Epigenetic Misregulation. Cells 9, (2020). https://doi.org/10.3390/cells9091968
Zhang, M., Li, Y., Rao, P., Huang, K., Luo, D., Cai, X., Xiao, J.: Blockade of receptors of advanced glycation end products ameliorates diabetic osteogenesis of adipose-derived stem cells through DNA methylation and Wnt signalling pathway. Cell Prolif. 51, 1–11 (2018). https://doi.org/10.1111/cpr.12471
Bröske, A.M., Vockentanz, L., Kharazi, S., Huska, M.R., Mancini, E., Scheller, M., Kuhl, C., Enns, A., Prinz, M., Jaenisch, R., Nerlov, C., Leutz, A., Andrade-Navarro, M.A., Jacobsen, S.E.W., Rosenbauer, F.: DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat. Genet. 41, 1207–1215 (2009). https://doi.org/10.1038/ng.463
Liu, C.C., Lin, J.H., Hsu, T.W., Su, K., Li, A.F.Y., Hsu, H.S., Hung, S.C.: IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int. J. Cancer 136, 547–559 (2015). https://doi.org/10.1002/ijc.29033
Morita, R., Hirohashi, Y., Suzuki, H., Takahashi, A., Tamura, Y., Kanaseki, T., Asanuma, H., Inoda, S., Kondo, T., Hashino, S., Hasegawa, T., Tokino, T., Toyota, M., Asaka, M., Torigoe, T., Sato, N.: DNA methyltransferase 1 is essential for initiation of the colon cancers. Exp. Mol. Pathol. 94, 322–329 (2013). https://doi.org/10.1016/j.yexmp.2012.10.004
He, J., Xu, Q., Jing, Y., Agani, F., Qian, X., Carpenter, R., Li, Q., Wang, X.R., Peiper, S.S., Lu, Z., Liu, L.Z., Jiang, B.H.: Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep. 13, 1116–1122 (2012). https://doi.org/10.1038/embor.2012.162
Zhang, L.N., Wang, X.X., Wang, Z., Li, K.Y., Xu, B.H., Zhang, J.: Berberine improves advanced glycation end products-induced osteogenic differentiation responses in human periodontal ligament stem cells through the canonical Wnt/β-catenin pathway. Mol. Med. Rep. 19, 5440–5452 (2019). https://doi.org/10.3892/mmr.2019.10193
Menini, S., Iacobini, C., de Latouliere, L., Manni, I., Ionta, V., Blasetti Fantauzzi, C., Pesce, C., Cappello, P., Novelli, F., Piaggio, G., Pugliese, G.: The advanced glycation end-product Nϵ-carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention. Journal of Pathology. 245, 197–208 (2018). https://doi.org/10.1002/path.5072
Geicu, O.I., Stanca, L., Voicu, S.N., Dinischiotu, A., Bilteanu, L., Serban, A.I., Calu, V.: Dietary AGEs involvement in colonic inflammation and cancer: insights from an in vitro enterocyte model. Sci. Rep. 10, (2020). https://doi.org/10.1038/s41598-020-59623-x
Peterson, L.L., Park, S., Park, Y., Colditz, G.A., Anbardar, N., Turner, D.P.: Dietary advanced glycation end products and the risk of postmenopausal breast cancer in the National Institutes of Health-AARP Diet and Health Study. Cancer 126, 2648–2657 (2020). https://doi.org/10.1002/cncr.32798
Roberts, M.J., Wondrak, G.T., Laurean, D.C., Jacobson, M.K., Jacobson, E.L.: DNA damage by carbonyl stress in human skin cells. Mutat. Res. 522, 45–56 (2003). https://doi.org/10.1016/s0027-5107(02)00232-4
Kuniyasu, H., Oue, N., Wakikawa, A., Shigeishi, H., Matsutani, N., Kuraoka, K., Ito, R., Yokozaki, H., Yasui, W.: Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 196, 163–170 (2002). https://doi.org/10.1002/PATH.1031
Nankali, M., Karimi, J., Goodarzi, M.T., Saidijam, M., Khodadadi, I., Razavi, A.N.E., Rahimi, F.: Increased Expression of the Receptor for Advanced Glycation End-Products (RAGE) Is Associated with Advanced Breast Cancer Stage. Oncology Research and Treatment. 39, 622–628 (2016). https://doi.org/10.1159/000449326
Kuniyasu, H., Chihara, Y., Kondo, H.: Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int. J. Cancer 104, 722–727 (2003). https://doi.org/10.1002/ijc.11016
Moy, K.A., Jiao, L., Freedman, N.D., Weinstein, S.J., Sinha, R., Virtamo, J., Albanes, D., Stolzenberg-Solomon, R.Z.: Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology 57, 2338–2345 (2013). https://doi.org/10.1002/hep.26264
Konopka, C.J., Woźniak, M., Hedhli, J., Siekierzycka, A., Skokowski, J., Pęksa, R., Matuszewski, M., Munirathinam, G., Kajdacsy-Balla, A., Dobrucki, I.T., Kalinowski, L., Dobrucki, L.W.: Quantitative imaging of the receptor for advanced glycation end-products in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging (2020). https://doi.org/10.1007/s00259-020-04721-1
Uribarri, J., Woodruff, S., Goodman, S., Cai, W., Chen, X., Pyzik, R., Yong, A., Striker, G.E., Vlassara, H.: Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 110, 911-916.e12 (2010). https://doi.org/10.1016/j.jada.2010.03.018
Peppa, M., Uribarri, J., Cai, W., Lu, M., Vlassara, H.: Glycoxidation and Inflammation in Renal Failure Patients. Am. J. Kidney Dis. 43, 690–695 (2004). https://doi.org/10.1053/j.ajkd.2003.11.022
Vlassara, H., Cai, W., Goodman, S., Pyzik, R., Yong, A., Chen, X., Zhu, L., Neade, T., Beeri, M., Silverman, J.M., Ferrucci, L., Tansman, L., Striker, G.E., Uribarri, J.: Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory age receptor-1. J. Clin. Endocrinol. Metab. 94, 4483–4491 (2009). https://doi.org/10.1210/jc.2009-0089
Chiappalupi, S., Sorci, G., Vukasinovic, A., Salvadori, L., Sagheddu, R., Coletti, D., Renga, G., Romani, L., Donato, R., Riuzzi, F.: Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J. Cachexia. Sarcopenia Muscle (2020). https://doi.org/10.1002/jcsm.12561
Nagai, R., Murray, D.B., Metz, T.O., Baynes, J.W.: Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 61, 549–559 (2012). https://doi.org/10.2337/DB11-1120
Jafarnejad, A., Bathaie, S.Z., Nakhjavani, M., Hassan, M.Z.: Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life Sci. 82, 301–307 (2008). https://doi.org/10.1016/j.lfs.2007.11.015
Kim, J., Jeong, I.H., Kim, C.S., Lee, Y.M., Kim, J.M., Kim, J.S.: Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharmacal Res. 34, 495–500 (2011). https://doi.org/10.1007/s12272-011-0319-5
Boor, P., Celec, P., Behuliak, M., Grančič, P., Kebis, A., Kukan, M., Pronayová, N., Liptaj, T., Ostendorf, T., Šebeková, K.: Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metab. Clin. Exp. 58, 1669–1677 (2009). https://doi.org/10.1016/j.metabol.2009.05.025
Delbin, M.A., Davel, A.P.C., Couto, G.K., de Araújo, G.G., Rossoni, L.V., Antunes, E., Zanesco, A.: Interaction between Advanced Glycation End Products Formation and Vascular Responses in Femoral and Coronary Arteries from Exercised Diabetic Rats. PLoS ONE 7, e53318 (2012). https://doi.org/10.1371/journal.pone.0053318
Macías-Cervantes, M.H., Rodríguez-Soto, J.M.D., Uribarri, J., Díaz-Cisneros, F.J., Cai, W., Garay-Sevilla, M.E.: Effect of an advanced glycation end product-restricted diet and exercise on metabolic parameters in adult overweight men. Nutrition 31, 446–451 (2015). https://doi.org/10.1016/j.nut.2014.10.004
Hsu, Y.H., Chen, S.Y., Wang, S.Y., Lin, J.A., Yen, G.C.: Pterostilbene enhances cytotoxicity and chemosensitivity in human pancreatic cancer cells. Biomolecules 10, (2020). https://doi.org/10.3390/biom10050709
Lin, J.H., Chen, S.Y., Lu, C.C., Lin, J.A., Yen, G.C.: Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in gemcitabine-resistant human pancreatic cancer cells. Phytother. Res. (2020). https://doi.org/10.1002/ptr.6669
Lan, C.Y., Chen, S.Y., Kuo, C.W., Lu, C.C., Yen, G.C.: Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 27, 887–896 (2019). https://doi.org/10.1016/j.jfda.2019.07.001
Guzmán, E.A., Pitts, T.P., Diaz, M.C., Wright, A.E.: The marine natural product Scalarin inhibits the receptor for advanced glycation end products (RAGE) and autophagy in the PANC-1 and MIA PaCa-2 pancreatic cancer cell lines. Invest. New Drugs 37, 262–270 (2019). https://doi.org/10.1007/s10637-018-0635-4
Liu, J., Huang, Y., Liu, Y., Chen, Y.: Irisin enhances doxorubicin-induced cell apoptosis in pancreatic cancer by inhibiting the PI3K/Akt/NF-kB pathway. Med. Sci. Monit. 25, 6085–6096 (2019). https://doi.org/10.12659/MSM.917625
Nakamara, N., Matsui, T., Ishibashi, Y., Sotokawauchi, A., Fukami, K., Higashimoto, Y., Yamagishi, S.I.: RAGE-aptamer attenuates the growth and liver metastasis of malignant melanoma in nude mice. Mol. Med. 23, 295–306 (2017). https://doi.org/10.2119/molmed.2017.00099
Ojima, A., Matsui, T., Maeda, S., Takeuchi, M., Inoue, H., Higashimoto, Y., Yamagishi, S.I.: DNA aptamer raised against advanced glycation end products inhibits melanoma growth in nude mice. Lab. Invest. 94, 422–429 (2014). https://doi.org/10.1038/labinvest.2014.5
Matsushita, S., Tada, K.I., Kawahara, K.I., Kawai, K., Hashiguchi, T., Maruyama, I., Kanekura, T.: Advanced malignant melanoma responds to Prunus mume sieb. Et Zucc (Ume) extract: Case report and in vitro study. Exp. Ther. Med. 1, 569–574 (2010). https://doi.org/10.3892/etm_00000089
Huang, H., Li, L., Zhang, H., Wei, A.: Papaverine selectively inhibits human prostate cancer cell ( PC-3 ) growth by inducing mitochondrial mediated apoptosis, cell cycle arrest and downregulation of NF-κB / PI3K / Akt signalling pathway. 22, 112–118 (2017)
Liu, Y., Gao, X., Deeb, D., Zhang, Y., Shaw, J., Valeriote, F.A., Gautam, S.C.: Mycotoxinverrucarin A inhibits proliferation and induces apoptosis in prostate cancer cells by inhibiting prosurvivalAkt/NF-kB/mTOR signaling. J. Exp. Ther. Oncol. 11, 251–260 (2016)
Hatashita, M., Taniguchi, M., Baba, K., Koshiba, K., Sato, T., Jujo, Y., Suzuki, R., Hayashi, S.: Sinodielide A exerts thermosensitizing effects and induces apoptosis and G2/M cell cycle arrest in DU145 human prostate cancer cells via the Ras/Raf/MAPK and PI3K/Akt signaling pathways. Int. J. Mol. Med. 33, 406–414 (2014). https://doi.org/10.3892/ijmm.2013.1568
Gao, X., Liu, Y., D., D.: Anticancer activity of pristimerin in ovarian carcinoma cells is mediated through the inhibition of prosurvival Akt/NF-κB/mTOR Signaling. J. Exp. Ther. Oncol. 10, 275–283 (2014)
Li, M.L., Wang, X.F., Tan, Z.J., Dong, P., Gu, J., Lu, J.H., Wu, X.S., Zhang, L., Ding, Q.C., Wu, W.G., Rao, L.H., Mu, J.S., Yang, J.H., Weng, H., Ding, Q., Zhang, W.J., Chen, L., Liu, Y.: bin: Ethyl pyruvate administration suppresses growth and invasion of gallbladder cancer cells via downregulation of HMGB1-RAGE axis. Int. J. Immunopathol. Pharmacol. 25, 955–965 (2012). https://doi.org/10.1177/039463201202500413
Takada M., Ku Y., Toyama H., Yasuyuki Suzuki, Y.K.: Suppressive effects of tea polyphenol and conformational changes with receptor for advanced glycation end products (RAGE) expression in human hepatoma cells - PubMed. Hepatogastroenterology 49(46), 928–31 (2002)
Sakuraoka, Y., Sawada, T., Okada, T., Shiraki, T., Miura, Y., Hiraishi, K., Ohsawa, T., Adachi, M., Takino, J.I., Takeuchi, M., Kubota, K.: MK615 decreases RAGE expression and inhibits tage-induced proliferation in hepatocellular carcinoma cells. World J. Gastroenterol. 16, 5334–5341 (2010). https://doi.org/10.3748/wjg.v16.i42.5334
Deperalta, D.K., Wei, L., Ghoshal, S., Schmidt, B., Lauwers, G.Y., Lanuti, M., Chung, R.T., Tanabe, K.K., Fuchs, B.C.: Metformin prevents hepatocellular carcinoma development by suppressing hepatic progenitor cell activation in a rat model of cirrhosis. Cancer 122, 1216–1227 (2016). https://doi.org/10.1002/cncr.29912
Yang, Y., Zhao, L.H., Huang, B., Wang, R.Y., Yuan, S.X., Tao, Q.F., Xu, Y., Sun, H.Y., Lin, C., Zhou, W.P.: Pioglitazone, a PPARγ agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol. Carcinog. 54, 1584–1595 (2015). https://doi.org/10.1002/mc.22231
Cheng, P., Dai, W., Wang, F., Lu, J., Shen, M., Chen, K., Li, J., Zhang, Y., Wang, C., Yang, J., Zhu, R., Zhang, H., Zheng, Y., Guo, C.Y., Xu, L.: Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE and AKT pathways. Biochem. Biophys. Res. Commun. 443, 1162–1168 (2014). https://doi.org/10.1016/j.bbrc.2013.12.064
Song, T.Y., Yang, N.C., Chen, C.L., Thi, T.L.V.: Protective effects and possible mechanisms of ergothioneine and hispidin against methylglyoxal-induced injuries in rat pheochromocytoma cells. Oxidative Med. Cell. Longev. 2017, (2017). https://doi.org/10.1155/2017/4824371
Jing, R., Chen, W., Wang, H., Ju, S., Cong, H., Sun, B., Jin, Q., Chu, S., Xu, L., Cui, M.: Plasma miR-185 is decreased in patients with esophageal squamous cell carcinoma and might suppress tumor migration and invasion by targeting RAGE. American Journal of Physiology - Gastrointestinal and Liver Physiology. 309, G719–G729 (2015). https://doi.org/10.1152/ajpgi.00078.2015
Tian, F., Fan, T., Zhang, Y.: Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo. Acta Biochim. Biophys. Sin. 44, 847–855 (2012). https://doi.org/10.1093/abbs/gms074
Xu, X.C., Zhang, W.B., Li, C.X., Gao, H., Pei, Q., Cao, B.W., He, T.H.: Up-Regulation of MiR-1915 inhibits proliferation, invasion, and migration of helicobacter pylori-infected gastric cancer cells via targeting RAGE. Yonsei Med. J. 60, 38–47 (2019). https://doi.org/10.3349/ymj.2019.60.1.38
Zhang, J., Zhu, J.S., Zhou, Z., Chen, W.X., Chen, N.W.: Inhibitory effects of ethyl pyruvate administration on human gastric cancer growth via regulation of the HMGB1-RAGE and Akt pathways in vitro and in vivo. (2012)
Yu, L.-L., Wu, J.-G., Dai, N., Yu, H.-G., Si, J.-M.: Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol. Rep. 26, 1197–1203 (2011). https://doi.org/10.3892/or.2011.1410
El-Far, A.H.A.M., Munesue, S., Harashima, A., Sato, A., Shindo, M., Nakajima, S., Inada, M., Tanaka, M., Takeuchi, A., Tsuchiya, H., Yamamoto, H., Shaheen, H.M.E., El-Sayed, Y.S., Kawano, S., Tanuma, S.I., Yamamoto, Y.: In vitro anticancer effects of a RAGE inhibitor discovered using a structure-based drug design system. Oncol. Lett. 15, 4627–4634 (2018). https://doi.org/10.3892/ol.2018.7902
Takeuchi, A., Yamamoto, Y., Munesue, S., Harashima, A., Watanabe, T., Yonekura, H., Yamamoto, H., Tsuchiya, H.: Low molecular weight heparin suppresses receptor for advanced glycation end products-mediated expression of malignant phenotype in human fibrosarcoma cells. Cancer Sci. 104, 740–749 (2013). https://doi.org/10.1111/cas.12133
Chonggao, Y.I.N., Zhang, G., Ruimei, S.U.N., Xinting, P.A.N., Wang, X., Hongli, L.I., Yunbo, S.U.N.: miR-185-5p inhibits F-actin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol. Med. Rep. 18, 2621–2630 (2018). https://doi.org/10.3892/mmr.2018.9294
Dhumale, S.S., Waghela, B.N., Pathak, C.: Quercetin protects necrotic insult and promotes apoptosis by attenuating the expression of RAGE and its ligand HMGB1 in human breast adenocarcinoma cells. IUBMB Life 67, 361–373 (2015). https://doi.org/10.1002/iub.1379
Ishibashi, Y., Matsui, T., Takeuchi, M., Yamagishi, S.: Metformin inhibits advanced glycation end products (AGEs)-induced growth and VEGF expression in MCF-7 breast cancer cells by suppressing AGEs receptor expression via AMP-activated protein kinase. Horm. Metab. Res. 45, 387–390 (2013). https://doi.org/10.1055/s-0032-1331204
Vinod, B.S., Antony, J., Nair, H.H., Puliyappadamba, V.T., Saikia, M., Shyam Narayanan, S., Bevin, A., John Anto, R.: Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 4, (2013). https://doi.org/10.1038/cddis.2013.26
Zhang, Z., Liu, W., Zheng, Y., Jin, L., Yao, W., Gao, X.: SGP-2, an acidic polysaccharide from Sarcandra glabra, inhibits proliferation and migration of human osteosarcoma cells. Food Funct. 5, 167–175 (2014). https://doi.org/10.1039/c3fo60378d
de Bittencourt Pasquali, M.A., Gelain, D.P., Zeidán-Chuliá, F., Pires, A.S., Gasparotto, J., Terra, S.R., Moreira, J.C.F.: Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell. Signal. 25, 939–954 (2013). https://doi.org/10.1016/j.cellsig.2013.01.013
Liu, Q., Huo, Y., Zheng, H., Zhao, J., Jia, L., Wang, P.: Ethyl pyruvate suppresses the growth, invasion and migration and induces the apoptosis of non-small cell lung cancer cells via the HMGB1/RAGE axis and the NF-κB/STAT3 pathway. Oncol. Rep. 42, 817–825 (2019). https://doi.org/10.3892/or.2019.7176
de Oliveira, M.R., Ferreira, G.C., Schuck, P.F., Dal Bosco, S.M.: Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem. Biol. Interact. 242, 396–406 (2015). https://doi.org/10.1016/j.cbi.2015.11.003
Sreekanth, C.N., Bava, S.V., Sreekumar, E., Anto, R.J.: Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene 30, 3139–3152 (2011). https://doi.org/10.1038/onc.2011.23
Wang, J., Zhong, S., Li, J., Du, W., Li, Y.: Scutellarein inhibits the development of colon cancer via CDC4-mediated RAGE ubiquitination. Int. J. Mol. Med. 45, 1059–1072 (2020). https://doi.org/10.3892/ijmm.2020.4496
Zheng, J., Zhu, W., He, F., Li, Z., Cai, N., Wang, H.H.: An Aptamer-Based Antagonist against the Receptor for Advanced Glycation End-Products (RAGE) Blocks Development of Colorectal Cancer. Mediat. Inflamm. 2021, (2021). https://doi.org/10.1155/2021/9958051
Acknowledgements
The authors acknowledge the Indian Council for Medical Research (ICMR), New Delhi, for providing Senior Research Fellowship to Yadav Sangeeta Muthyalaiah
Funding
No specific funding was received for writing this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muthyalaiah, Y.S., Jonnalagadda, B., John, C.M. et al. Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression. Glycoconj J 38, 717–734 (2021). https://doi.org/10.1007/s10719-021-10031-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-021-10031-x