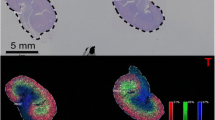
Abstract
Glycans play an important physiological role and are drawing attention as biomarkers that capture pathophysiological changes. Glycans can be detected by mass spectrometry, but recently matrix-assisted laser desorption/ionization- mass spectrometry imaging (MALDI-MSI) has enabled the visualization of glycans distribution on tissues. In this study, focusing on sialylated glycan (sialoglycans), we investigated the amidation reaction used to visualize glycans distribution, and developed a method of sialic acid derivatization by benzylamidation which is more sensitive than conventional amidation. Furthermore, we adapted this method for visualizing glycans in formalin-fixed paraffin-embedded (FFPE) liver tissue from normal mice and non-alcoholic steatohepatitis (NASH) model mice using MALDI-MSI. As a result, an increase in the distribution of glycan N-Acetylneuraminic acid-(NeuAc) ions was observed in the NASH mouse liver, and the change in glycan structure in the NASH model was clarified.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MALDI -MSI:
-
matrix-assisted laser desorption/ionization- mass spectrometry imaging
- FFPE:
-
formalin-fixed paraffin-embedded
- NASH:
-
non-alcoholic steatohepatitis
- NeuAc:
-
N-Acetylneuraminic acid
- NeuGc:
-
N-glycolylneuraminic acids
- DHB:
-
dihydroxybenzoic acid
- EDC:
-
1-ethyl-3-(3- (dimethylamino)propyl) carbodiimide
- PyAOP:
-
(7-Azabenzotriazol-1-yloxy) tripyrrolidinophosphonium hexafluorophosphate
References
Varki A, Cummings RD, Esko JD, et al., Essentials of Glycobiology 3rd edition. Cold Spring Harbor (NY): 2015-2017
Nakano, M., Saldanha, R., Göbel, A., Kavallaris, M., Packer, N.H.: Identification of glycan structure alterations on cell membrane proteins in desoxyepothilone B resistant leukemia cells. Mol. Cell. Proteomics. 10, M111.009001 (2011)
Powers, T.W., Jones, E.E., Betesh, L.R., Romano, P.R., Gao, P., Copland, J.A., Mehta, A.S., Drake, R.R.: Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked glycan expression in tissues. Anal. Chem. 85, 9799–9806 (2013)
Powers, T.W., Neely, B.A., Shao, Y., Tang, H., Troyer, D.A., Mehta, A.S., Haab, B.B., Drake, R.R.: MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS One. 9, e106255 (2014)
Gustafsson, O.J., Briggs, M.T., Condina, M.R., Winderbaum, L.J., Pelzing, M., McColl, S.R., Everest-Dass, A.V., Packer, N.H., Hoffmann, P.: MALDI imaging mass spectrometry of N-linked glycans on formalin-fixed paraffin-embedded murine kidney. Anal. Bioanal. Chem. 407, 2127–2139 (2015)
Kang, P., Mechref, Y., Klouckova, I., Novotny, M.V.: Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 19, 3421–3428 (2005)
Zaia, J.: Mass spectrometry and Glycomics. OMICS. 14, 401–418 (2010)
Reiding, K.R., Lonardi, E., Hipgrave Ederveen, A.L., Wuhrer, M.: Ethyl esterification for MALDI-MS analysis of protein glycosylation. Methods Mol. Biol. 394, 151–162 (2016)
Toyoda, M., Ito, H., Matsuno, Y.K., Narimatsu, H., Kameyama, A.: Quantitative derivatization of sialic acids for the detection of sialoglycans by MALDI MS. Anal. Chem. 80, 5211–5218 (2008)
de Haan, N., Reiding, K.R., Haberger, M., Reusch, D., Falck, D., Wuhrer, M.: Linkage-specific sialic acid derivatization for MALDI-TOF-MS profiling of IgG glycopeptides. Anal. Chem. 87, 8284–8291 (2015)
Nishikaze, T., Tsumoto, H., Sekiya, S., Iwamoto, S., Miura, Y., Tanaka, K.: Differentiation of Sialyl linkage isomers by one-pot Sialic acid Derivatization for mass spectrometry-based glycan profiling. Anal. Chem. 89, 2353–2360 (2017)
Holst, S., Heijs, B., de Haan, N., van Zeijl, R.J., Briaire-de Bruijn, I.H., van Pelt, G.W., Mehta, A.S., Angel, P.M., Mesker, W.E., Tollenaar, R.A., Drake, R.R., Bovée, J.V., McDonnell, L.A., Wuhrer, M.: Linkage-specific in situ Sialic acid Derivatization for N-glycan mass spectrometry imaging of formalin-fixed paraffin-embedded tissues. Anal. Chem. 88, 5904–5913 (2016)
Matsumoto, M., Hada, N., Sakamaki, Y., Uno, A., Shiga, T., Tanaka, C., Ito, T., Katsume, A., Sudoh, M.: An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103 (2013)
Wilson, N.L., Schulz, B.L., Karlsson, N.G., Packer, N.H.: Sequential analysis of N- and O-linked glycosylation of 2D-PAGE separated glycoproteins. J. Proteome Res. 1, 521–529 (2002)
Green, E.D., Adelt, G., Baenziger, J.U., Wilson, S., Van Halbeek, H.: The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J. Biol. Chem. 263, 18253–18268 (1988)
Townsend, R.R., Hardy, M.R., Hindsgaul, O., Lee, Y.C.: High-performance anion-exchange chromatography of oligosaccharides using pellicular resins and pulsed amperometric detection. Anal. Biochem. 174, 459–470 (1988)
Nakano, M., Nakagawa, T., Ito, T., Kitada, T., Hijioka, T., Kasahara, A., Tajiri, M., Wada, Y., Taniguchi, N., Miyoshi, E.: Site-specific analysis of N-glycans on haptoglobin in sera of patients with pancreatic cancer: a novel approach for the development of tumor markers. Int. J. Cancer. 122, 2301–2309 (2008)
Author information
Authors and Affiliations
Contributions
M.N. and K.M. conceived and designed the experiments; T.S. and A.W. performed the experiments; T.S., A.W., M.N., and K.M. analyzed the data; T.S., M.N., and K.M. wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saito, T., Watanabe, A., Nakano, M. et al. MALDI-TOF mass spectrometry imaging for N-glycans on FFPE tissue sections of mouse NASH liver through Sialic acid Benzylamidation. Glycoconj J 38, 167–175 (2021). https://doi.org/10.1007/s10719-021-09984-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-021-09984-w