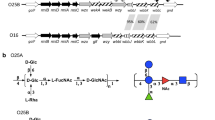
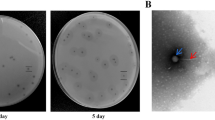
Abstract
Enterococcus faecium (E. faecium) has emerged as one of today’s leading causes of health care-associated infections that is difficult to treat with the available antibiotics. These pathogens produce capsular polysaccharides on the cell surface which play a significant role in adhesion, virulence and evasion. Therefore, we aimed at the identification and characterization of bacterial polysaccharide antigens which are central for the development of vaccine-based prophylactic approaches. The crude cell wall-associated polysaccharides from E. faecium, its mutant and complemented strains were purified and analyzed by a primary antibody raised against lipoteichoic acid (LTA) and diheteroglycan (DHG). The resistant E. faecium strains presumably possess novel capsular polysaccharides that allow them to avoid the evasion from opsonic killing. The E. faecium U0317 strain was very well opsonized by anti-U0317 (~95%), an antibody against the whole bacterial cell. The deletion mutant showed a significantly increased susceptibility to opsonophagocytic killing (90–95%) against the penicillin binding protein (anti-PBP-5). By comparison, in a mouse urinary tract and rat endocarditis infection model, respectively, there were no significant differences in virulence. In this study we explored the biological role of the capsule of E. faecium. Our findings showed that the U0317 strain is not only sensitive to anti-LTA but also to antibodies against other enterococcal surface proteins. Our findings demonstrate that polysaccharides capsule mediated-resistance to opsonophagocytosis. We also found that the capsular polysaccharides do not play an important role in bacterial virulence in urinary tract and infective endocarditis in vivo models.




Similar content being viewed by others
Abbreviations
- CPS :
-
Capsular polysaccharides
- CFU :
-
Colony-forming unit
- DHG :
-
Diheteroglycan
- E. faecium :
-
Enterococcus faecium
- LTA :
-
Lipoteichoic Acid
- OPA :
-
Opsonophagocytic Assays
- PBP :
-
Penicillin Binding Protein-5
- UTI :
-
Urinary Tract Infection
- VRE :
-
Vancomycin-Resistant Enterococci
- Wt :
-
Wild-type
References
Costerton, J.W., Irvin, R.T., Cheng, K.J.: The bacterial glycocalyx in nature and disease. Annu. Rev. Microbiol. 35, 299–324 (1981)
Micoli, F., Costantino, P., Adamo, R.: Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 42, 388–423 (2018)
Whitfield, C., Valvano, M.A.: Biosynthesis and expression of cell-surface polysaccharides in gram-negative bacteria. Adv. Microb. Physiol. 35, 135–246 (1993)
Boulnois, G.J., Roberts, I.S.: Genetics of capsular polysaccharide production in bacteria. Curr. Top. Microbiol. Immunol. 150, 1–18 (1990)
O’Riordan, K., Lee, J.C.: Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17, 218–234 (2004)
Kadioglu, A., Weiser, J.N., Paton, J.C., Andrew, P.W.: The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6, 288–301 (2008)
Enterococci: From commensals to leading causes of drug resistant infection - PubMed - NCBI, (2014)
Thurlow, L.R., Thomas, V.C., Hancock, L.E.: Capsular polysaccharide production in Enterococcus faecalis and contribution of CpsF to capsule serospecificity. J. Bacteriol. 191, 6203–6210 (2009)
McBride, S.M., Fischetti, V.A., Leblanc, D.J., Moellering, R.C., Gilmore, M.S.: Genetic diversity among Enterococcus faecalis. PLoS One. 2, e582 (2007)
Thurlow, L.R., Thomas, V.C., Fleming, S.D., Hancock, L.E.: Enterococcus faecalis capsular polysaccharide serotypes C and D and their contributions to host innate immune evasion. Infect. Immun. 77, 5551–5557 (2009)
Ali, L., Spiess, M., Wobser, D., Rodriguez, M., Blum, H.E., Sakιnç, T.: Identification and functional characterization of the putative polysaccharide biosynthesis protein (CapD) of Enterococcus faecium U0317. Infect. Genet. Evol. 37, 215–224 (2016)
Bentley, S.D., Aanensen, D.M., Mavroidi, A., Saunders, D., Rabbinowitsch, E., Collins, M., Donohoe, K., Harris, D., Murphy, L., Quail, M.A., Samuel, G., Skovsted, I.C., Kaltoft, M.S., Barrell, B., Reeves, P.R., Parkhill, J., Spratt, B.G.: Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31 (2006)
Pan, Y.-J., Lin, T.-L., Chen, C.-T., Chen, Y.-Y., Hsieh, P.-F., Hsu, C.-R., Wu, M.-C., Wang, J.-T.: Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci. Rep. 5, 15573 (2015)
Palmer, K.L., Godfrey, P., Griggs, A., Kos, V.N., Zucker, J., Desjardins, C., Cerqueira, G., Gevers, D., Walker, S., Wortman, J., Feldgarden, M., Haas, B., Birren, B., Gilmore, M.S.: Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio. 3, e00318–e00311 (2012)
Huebner, J., Wang, Y., Krueger, W.A., Madoff, L.C., Martirosian, G., Boisot, S., Goldmann, D.A., Kasper, D.L., Tzianabos, A.O., Pier, G.B.: Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect. Immun. 67, 1213–1219 (1999)
Theilacker, C., Kaczynski, Z., Kropec, A., Fabretti, F., Sange, T., Holst, O., Huebner, J.: Opsonic antibodies to Enterococcus faecalis strain 12030 are directed against lipoteichoic acid. Infect. Immun. 74, 5703–5712 (2006)
Theilacker, C., Kaczyński, Z., Kropec, A., Sava, I., Ye, L., Bychowska, A., Holst, O., Huebner, J.: Serodiversity of opsonic antibodies against Enterococcus faecalis--glycans of the cell wall revisited. PLoS One. 6, e17839 (2011)
Qin, X., Galloway-Peña, J.R., Sillanpaa, J., Roh, J.H., Nallapareddy, S.R., Chowdhury, S., Bourgogne, A., Choudhury, T., Muzny, D.M., Buhay, C.J., Ding, Y., Dugan-Rocha, S., Liu, W., Kovar, C., Sodergren, E., Highlander, S., Petrosino, J.F., Worley, K.C., Gibbs, R.A., Weinstock, G.M., Murray, B.E.: Complete genome sequence of Enterococcus faecium strain TX16 and comparative genomic analysis of Enterococcus faecium genomes. BMC Microbiol. 12, 135 (2012)
Laverde, D., Wobser, D., Romero-Saavedra, F., Hogendorf, W., van der Marel, G., Berthold, M., Kropec, A., Codee, J., Huebner, J.: Synthetic teichoic acid conjugate vaccine against nosocomial gram-positive bacteria. PLoS One. 9, e110953 (2014)
Kodali, S., Vinogradov, E., Lin, F., Khoury, N., Hao, L., Pavliak, V., Jones, C.H., Laverde, D., Huebner, J., Jansen, K.U., Anderson, A.S., Donald, R.G.K.: A vaccine approach for the prevention of infections by multidrug-resistant Enterococcus faecium. J. Biol. Chem. 290, 19512–19526 (2015)
van Schaik, W., Top, J., Riley, D.R., Boekhorst, J., Vrijenhoek, J.E.P., Schapendonk, C.M.E., Hendrickx, A.P.A., Nijman, I.J., Bonten, M.J.M., Tettelin, H., Willems, R.J.L.: Pyrosequencing-based comparative genome analysis of the nosocomial pathogen Enterococcus faecium and identification of a large transferable pathogenicity island. BMC Genomics. 11, 239 (2010)
Turula, V.E., Gore, T., Singh, S., Arumugham, R.G.: Automation of the anthrone assay for carbohydrate concentration determinations. Anal. Chem. 82, 1786–1792 (2010)
Dische, Z., Shettles, L.B.: A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. Biol. Chem. 175, 595–603 (1948)
Lowry, O.H., Roberts, N.R., WU, M.L., Hixon, W.S., Crawford, E.J.: The quantitative histochemistry of brain. II. Enzyme measurements. J. Biol. Chem. 207, 19–37 (1954)
Kropec, A., Sava, I.G., Vonend, C., Sakinc, T., Grohmann, E., Huebner, J.: Identification of SagA as a novel vaccine target for the prevention of Enterococcus faecium infections. Microbiology. 157, 3429–3434 (2011)
Haller, C., Berthold, M., Wobser, D., Kropec, A., Lauriola, M., Schlensak, C., Huebner, J.: Cell-wall glycolipid mutations and their effects on virulence of E. faecalis in a rat model of infective endocarditis. PLoS One. 9, e91863 (2014)
Gründling, A., Schneewind, O.: Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J. Bacteriol. 189, 2521–2530 (2007)
Romero-Saavedra, F., Laverde, D., Wobser, D., Michaux, C., Budin-Verneuil, A., Bernay, B., Benachour, A., Hartke, A., Huebner, J.: Identification of peptidoglycan-associated proteins as vaccine candidates for enterococcal infections. PLoS One. 9, e111880 (2014)
Sava, I.G., Heikens, E., Huebner, J.: Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 16, 533–540 (2010)
Wobser, D., Ali, L., Grohmann, E., Huebner, J., Sakinc, T.: A novel role for D-alanylation of lipoteichoic acid of enterococcus faecalis in urinary tract infection. PLoS One. 9, e107827 (2014)
Theilacker, C., Sanchez-Carballo, P., Toma, I., Fabretti, F., Sava, I., Kropec, A., Holst, O., Huebner, J.: Glycolipids are involved in biofilm accumulation and prolonged bacteraemia in Enterococcus faecalis. Mol. Microbiol. 71, 1055–1069 (2009)
Diederich, A.-K., Wobser, D., Spiess, M., Sava, I.G., Huebner, J., Sakιnç, T.: Role of glycolipids in the pathogenesis of Enterococcus faecalis urinary tract infection. PLoS One. 9, e96295 (2014)
Paganelli, F.L., Huebner, J., Singh, K.V., Zhang, X., van Schaik, W., Wobser, D., Braat, J.C., Murray, B.E., Bonten, M.J.M., Willems, R.J.L., Leavis, H.L.: Genome-wide screening identifies phosphotransferase system permease BepA to be involved in Enterococcus faecium endocarditis and biofilm formation. J. Infect. Dis. 214, 189–195 (2016)
Fabretti, F., Theilacker, C., Baldassarri, L., Kaczynski, Z., Kropec, A., Holst, O., Huebner, J.: Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect. Immun. 74, 4164–4171 (2006)
Weidenmaier, C., Peschel, A., Xiong, Y.-Q., Kristian, S.A., Dietz, K., Yeaman, M.R., Bayer, A.S.: Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191, 1771–1777 (2005)
Neuhaus, F.C., Baddiley, J.: A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 686–723 (2003)
Morath, S., Stadelmaier, A., Geyer, A., Schmidt, R.R., Hartung, T.: Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195, 1635–1640 (2002)
Weintraub, A.: Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 338, 2539–2547 (2003)
Ada, G.: Vaccines and vaccination. N. Engl. J. Med. 345, 1042–1053 (2001)
Weidenmaier, C., Peschel, A.: Teichoic acids and related cell-wall glycopolymers in gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6, 276–287 (2008)
Roberts, I.S.: The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50, 285–315 (1996)
Roy, D., Auger, J.-P., Segura, M., Fittipaldi, N., Takamatsu, D., Okura, M., Gottschalk, M.: Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 79, 141–146 (2015)
Geiss-Liebisch, S., Rooijakkers, S.H.M., Beczala, A., Sanchez-Carballo, P., Kruszynska, K., Repp, C., Sakinc, T., Vinogradov, E., Holst, O., Huebner, J., Theilacker, C.: Secondary cell wall polymers of Enterococcus faecalis are critical for resistance to complement activation via mannose-binding lectin. J. Biol. Chem. 287, 37769–37777 (2012)
Thurman, J.M., Holers, V.M.: The central role of the alternative complement pathway in human disease. J. Immunol. 176, 1305–1310 (2006)
Kleine, B., Ali, L., Wobser, D., Sakιnç, T.: The N-terminal repeat and the ligand binding domain a of SdrI protein is involved in hydrophobicity of S. saprophyticus. Microbiol. Res. 172, 88–94 (2015)
Nicolle, L.E.: Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 3(23), (2014)
Shokoohizadeh, L., Mobarez, A.M., Zali, M.R., Ranjbar, R., Alebouyeh, M., Sakinc, T., Ali, L.: High frequency distribution of heterogeneous vancomycin resistant Enterococcous faecium (VREfm) in Iranian hospitals. Diagn. Pathol. 8(902), (2013)
Shankar, N., Lockatell, C.V., Baghdayan, A.S., Drachenberg, C., Gilmore, M.S., Johnson, D.E.: Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect. Immun. 69, 4366–4372 (2001)
Kau, A.L., Martin, S.M., Lyon, W., Hayes, E., Caparon, M.G., Hultgren, S.J.: Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73, 2461–2468 (2005)
Wang, A., Athan, E., Pappas, P.A., Fowler, V.G., Olaison, L., Paré, C., Almirante, B., Muñoz, P., Rizzi, M., Naber, C., Logar, M., Tattevin, P., Iarussi, D.L., Selton-Suty, C., Jones, S.B., Casabé, J., Morris, A., Corey, G.R., Cabell, C.H.: International collaboration on endocarditis-prospective cohort study investigators: contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 297, 1354–1361 (2007)
Donlan, R.M., Costerton, J.W.: Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193 (2002)
Acknowledgments
LA sincerely thanks the Deutscher Akademischer AustauschDienst (DAAD) for the award of a PhD fellowship and Dominique Wobser for help with the animal experiments.
Funding
This work was supported by grants from the German Ministry of Science and Education (BMBF: UroGenOmics0315833C).
Author information
Authors and Affiliations
Contributions
LA and TS conceived and designed the study. LA analyzed the data and drafted the manuscript. HEB critically reviewed the manuscript. All the authors studied and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All animal experiments were performed in compliance with the German animal protection law (TierSchG). The mice were housed and handled following good animal practice as defined by FELASA and the national animal welfare body GV-SOLAS. The animal welfare committees of the University of Freiburg (Regierungspräsidium Freiburg Az 35/9185.81/G-11/118 and Az 35/9185.81/G-12/070) approved all animal experiments.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, L., Blum, H.E. & Sakιnç, T. Detection and characterization of bacterial polysaccharides in drug-resistant enterococci. Glycoconj J 36, 429–438 (2019). https://doi.org/10.1007/s10719-019-09881-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-019-09881-3