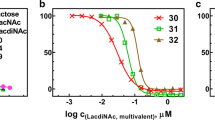
Abstract
Galectin-3 is associated with the development and malignancy of several types of tumor, mediating important tumor-related functions, such as tumorigenesis, neoplastic transformation, tumor cell survival, angiogenesis, tumor metastasis and regulation of apoptosis. Therefore, synthetic galectin-3 inhibitors are of utmost importance for development of new antitumor therapeutic strategies. In this review we present an updated selection of synthetic glycoconjugates inhibitors of tumor-related galectin-3, properly addressed as monosaccharide- and disaccharide-based inhibitors, and multivalent-based inhibitors, disclosuring relevant methods for their synthesis along with their inhibitory activities towards galectin-3. In general, Cu(I)-assisted 1,3-dipolar azide-alkyne cycloaddition (CuAAC) reactions were predominantly applied for the synthesis of the described inhibitors, which had their inhibitory activities against galectin-3 evaluated by fluorescence polarization, surface plasmon resonance (SPR), hemagglutination, ELISA and cell imaging assays. Overall, the presented synthetic glycoconjugates represent frontline galectin-3 inhibitors, finding important biomedical applications in cancer.

























Similar content being viewed by others
References
Boligan K.F., Mesa C., Fernandez L.E., von Gunten S.: Cancer intelligence acquired (CIA): tumor glycosylation and sialylation codes dismantling antitumor defense. Cell. Mol. Life Sci. 72, 1231–1248 (2015)
Dall'Olio F., Malagolini N., Trinchera M., Chiricolo M.: Mechanisms of cancer-associated glycosylation changes. Front. Biosci. (Landmark Ed). 17(670–699), (2012)
Stowell S.R., Ju T., Cummings R.D.: Protein glycosylation in cancer. Annu. Rev. Pathol. 10, 473–510 (2015)
Nakahara S., Raz A.: Biological modulation by lectins and their ligands in tumor progression and metastasis. Anti Cancer Agents Med. Chem. 8, 22–36 (2008)
Boscher C., Dennis J.W., Nabi I.R.: Glycosylation, galectins and cellular signaling. Curr. Opin. Cell Biol. 23, 383–392 (2011)
Garner O.B., Baum L.G.: Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem. Soc. Trans. 36, 1472–1477 (2008)
Hockl P.F., Wolosiuk A., Sáez J.M., Bordoni A.V., Croci D.O., Terrones Y.T., Illia G.J., Rabinovich G.A.: Glyco-nano-oncology: Novel therapeutic opportunities by combining small and sweet. Pharmacol. (2016). doi:10.1016/j.phrs.2016.02.005
Ahmed H., AlSadek D.M.: Galectin-3 as a potential target to prevent cancer metastasis. Clin. Med. Insights Oncol. 25, 113–121 (2015)
Arthur, C. M.; Baruffi, M. D.; Cummings, R. D.; Stowell, S. R.: Galectins: methods and protocols. Stowell, S. R.; Cummings, R. D. (eds.); human press, Vol. 1, Chapter 1, pp 1–35 (2015)
Marchiori M.F., Souto D.E., Bortot L.O., Pereira J.F., Kubota L.T., Cummings R.D., Baruffi M.D., Carvalho I., Campo V.L.: Synthetic 1,2,3-triazole-linked glycoconjugates bind with high affinity to human galectin-3. Bioorg. Med. Chem. 23, 3414–3425 (2015)
van den Brüle F., Califice S., Castronovo V.: Expression of galectins in cancer: a critical review. Glycoconj. J. 19, 537–542 (2004)
Liu F.T., Rabinovich G.A.: Galectins as modulators of tumour progression. Nat. Rev. Cancer. 5, 29–41 (2005)
Califice S., Castronovo V., Bracke M., et al.: Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 23, 7527–7536 (2004)
Sun W., Li L., Yang Q., Shan W., Zhang Z., Huang Y.: G3-C12 peptide reverses galectin-3 from foe to friend for active targeting cancer treatment. Mol. Pharm. 12, 4124–4136 (2015)
Cecchinelli B., Lavra L., Rinaldo C., Iacovelli S., Gurtner A., Gasbarri A., Ulivieri A., Del Prete F., Trovato M., Piaggio G., Bartolazzi A., Soddu S., Sciacchitano S.: Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol. Cell. Biol. 26, 4746–4757 (2006)
Yang R.Y., Rabinovich G.A., Liu F.T.: Galectins: structure, function and therapeutic potential. Expert. Rev. Mol. Med. (2008). doi:10.1017/S1462399408000719
Leffler H., Carlsson S., Hedlund M., Qian Y., Poirier F.: Introduction to galectins. Glycoconj. J. 19, 433–440 (2004)
Knibbs R.N., Agrwal N., Wang J.L., Goldstein I.J.: Carbohydrate-binding protein 35. II. Analysis of the interaction of the recombinant polypeptide with saccharides. J. Biol. Chem. 268, 14940–14947 (1993)
Tejler J., Salameh B., Lefflerc H., Nilsson U.J.: Fragment-based development of triazole-substituted O-galactosyl aldoximes with fragment-induced affinity and selectivity for galectin-3. J. Org. Biomol. Chem. 7, 3982–3990 (2009)
Bum-Erdene K., Gagarinov I.A., Collins P.M., Winger M., Pearson A.G., Wilson J.C., Leffler H., Nilsson U.J., Grice I.D., Blanchard H.: Investigation into the feasibility of thioditaloside as a novel scaffold for galectin-3-specific inhibitors. Chembiochem. 14, 1331–1339 (2013)
Stowell S.R., Karmakar S., Stowell C.J., Baruffi M.D., McEver R.P., Cummings R.D.: Human galectin-1, −2, and −4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 109, 219–227 (2007)
Rapoport E.M., Kurmyshkina O.V., Bovin N.V.: Mammalian galectins: structure, carbohydrate specificity, and functions. Biochem. Mosc. 73, 393–405 (2008)
Giguere D., Andre S., Bonin M.A., Bellefleur M.A., Provençal A., Cloutier P., Pucci B., Roy R., Gabius H.J.: Inhibitory potential of chemical substitutions at bioinspired sites of β-D-galactopyranose on neoglycoprotein/cell surface binding of two classes of medically relevant lectins. J. Bioorg. Med. Chem. Lett. 19, 3280–3287 (2011)
Cumpstey I., Sundin A., Leffler H., Nilsson U.J.: C2-symmetrical thiodigalactoside bis-benzamido derivatives as high-affinity inhibitors of galectin-3: efficient lectin inhibition through double arginine-arene interactions. J. Angew. Chem. Int. Ed. 44, 5110–5112 (2005)
Cumpstey I., Salomonsson E., Sundin A., Leffler H., Nilsson U.J.: Double affinity amplification of galectin-ligand interactions through arginine-arene interactions: synthetic, thermodynamic, and computational studies with aromatic diamido thiodigalactosides. Chem. 14, 4233–4245 (2008)
Aragão-Leoneti V., Campo V.L., Gomes A.S., Field R.A., Carvalho I.: Application of copper(I)-catalysed azide/alkyne cycloaddition (CuAAC) ‘click chemistry’ in carbohydrate drug and neoglycopolymer synthesis. Tetrahedron. 66, 9475–9492 (2010)
Tiwari V.K., Mishra B.B., Mishra K.B., Mishra N., Singh A.S., Chen X.: Cu-catalyzed click reaction in carbohydrate chemistry. Chem. Rev. 116, 3086–3240 (2016)
Blanchard H., Yu X., Collins P.M., Bum-Erdene K.: Galectin-3 inhibitors: a patent review (2008-present). Expert Opin. Ther. Pat. 24, 1053–1065 (2008)
Téllez-Sanz R., García-Fuentes L., Vargas-Berenguel A.: Human galectin-3 selective and high affinity inhibitors. Present state and future perspectives. Curr. Med. Chem. 20, 2979–2990 (2013)
Yang R.-Y., Rabinovich G.A., Liu F.-T.: Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 10, e17 (2008)
Nakahara S., Raz A.: Biological Modulation by lectins and their ligands in tumor progression and metastasis. Anti Cancer Agents Med. Chem. 8, 22–36 (2008)
Klyosov A.A., Traber P.G.: Galectins and disease implications for targeted therapeutics. ACS Symposium Series, American Chemical Society, Washington (2012)
Radosavljevic G.D., Pantic J., Jovanovic I., Lukic M.L., Arsenijevic N.: The two faces of galectin-3: roles in various pathological conditions. Ser. J. Exp. Clin. Res. 17, 1–1 (2016)
Giguére D., Patnam R., Bellefleur M.-A., St-Pierre C., Sato S., Roy R.: Carbohydrate triazoles and isoxazoles as inhibitors of galectins-1 and −3. Chem. Commun. 14, 2379–2381 (2006)
Tejler J., Skogman F., Lefflerc H., Nilsson U.J.: Synthesis of galactose-mimicking 1H-(1,2,3-triazol-1-yl)- mannosides as selective galectin-3 and 9 N inhibitors. Carbohydr. Res. 342, 1869–1875 (2007)
Compagno D., Jaworski F.M., Gentilini L., Contrufo G., González Pérez I., Elola M.T., Pregi N., Rabinovich G.A., Laderach D.J.: Galectins: major signaling modulators inside and outside the cell. Curr. Mol. Med. 14, 630–651 (2014)
Bum-Erdene K., Gagarinov I.A., Collins P.M., Winger M., Pearson A.G., Wilson J.C., Leffler H., Nilsson U.J., Grice I.D., Blanchard H.: Investigation into the feasibility of thioditaloside as a novel scaffold for galectin-3-specific inhibitors. ChemBiolChem. 14, 1331–1342 (2013)
Salameh B.A., Cumpstey I., Sundin A., Leffler H., Nilsson U.J.: 1H-1,2,3-triazol-1-yl thiodigalactoside derivatives as high affinity galectin-3 inhibitors. Bioorg. Med. Chem. 18, 5367–5378 (2010)
Fort S., Kim H.-S., Hindsgaul O.: Screening for galectin-3 inhibitors from synthetic lacto-N-biose libraries using microscale affinity chromatography coupled to mass spectrometry. J. Organomet. Chem. 71, 7146–7154 (2006)
Öberg C., Blanchard H., Leffler H., Nilsson U.J.: Protein subtype-targeting through ligand epimerization: talose-selectivity of galectin-4 and galectin-8. Bioorg. Med. Chem. Lett. 18, 3691–3694 (2008)
Öberg C., Noresson A.-L., Leffler H., Nilsson U.J.: Synthesis of 3-amido-3-deoxy-beta-D-talopyranosides: all-cis-substituted pyranosides as lectin inhibitors. Tetrahedron. 67, 9164–9172 (2011)
Collins P.M., Oberg C.T., Leffler H., Nilsson U.J., Blanchard H.: Taloside inhibitors of galectin-1 and galectin-3. Chem. Biol. Drug Des. 79, 339–346 (2012)
van Hattum H., Branderhorst H.M., Moret E.E., Nilsson U.J., Leffler H., Pieters R.J.: Tuning the preference of thiodigalactoside- and lactosamine-based ligands to galectin-3 over galectin-1. J. Med. Chem. 56, 1350–1354 (2013)
Cumpstey I., Solomonsson E., Sundin A., Leffler H., Nilsson U.J.: Double affinity amplification of galectin–ligand interactions through arginine–arene interactions: synthetic, thermodynamic, and computational studies with aromatic diamido thiodigalactosides. Chem. Eur. J. 14, 4233–4245 (2008)
Öberg C.T., Leffler H., Nilsson U.J.: Arginine binding motifs: design and synthesis of galactose-derived arginine tweezers as galectin-3 inhibitors. J. Med. Chem. 51, 2297–2301 (2008)
Öberg C.T., Leffler H., Nilsson U.J.: Inhibition of galectins with small molecules. Chimia. 65, 18–23 (2011)
Guigere D., Sato S., St-Pierre C., Sirois S., Roy R.: Aryl O- and S-galactosides and lactosides as specific inhibitors of human galectins-1 and −3: role of electrostatic potential at O-3. Bioorg. Med. Chem. Lett. 16, 1668–1672 (2006)
Rauthu S.R., Shiao T.C., André S., Miller M.C., Madej E., Mayo K.H., Gabius H.-J., Roy R.: Defining the potential of aglycone modifications for affinity/selectivity enhancement against medicallyrelevant lectins: synthesis, activity screening, and HSQCBased NMR analysis. Chembiochem. 16, 126–139 (2015)
Bianchet M.A., Ahmed H., Vasta G.R., Amzel L.M.: Soluble β-galactosyl-binding lectin (galectin) from toad ovary: crystallographic studies of two protein-sugar complexes. Proteins: Struct., Funct., Bioinf. 40, 378–388 (2002)
Pieters R.J.: Inhibition and Detection of Galectins. ChemBioChem. 7, 721–728 (2006)
van Scherpen zeel M., Moret E.E., Ballell L., Liskamp R.M.J., Nilsson U.J., Leffler H., Pieters R.J.: Synthesis and Evaluation of New Thiodigalactoside-Based Chemical Probes to Label Galectin-3. ChemBioChem. 10, 1724–1733 (2009)
André S., Kövér K.E., Gabius H.-J., Szilágyi L.: Thio- and selenoglycosides as ligands for biomedically relevant lectins: Valency–activity correlations for benzene-based dithiogalactoside clusters and first assessment for (di)selenodigalactosides. Bioorg. Med. Chem. Lett. 25, 931–935 (2015)
Wagner G., Nuhn P.: Synthese von Selenoglykosiden mit Acetyl-glykosyl-isoselenuronium-bromiden. Arch. Pharm. 297, 461–473 (1964)
Galante E., Geraci C., Sciuto S., Campo V.L., Carvalho I., Sesti-Costa R., Guedes P.M.M., Silva J.S., Hill L., Nepogodiev S.A., Field R.A.: Glycoclusters presenting lactose on calix[4]arene cores display trypanocidal activity. Tetrahedron. 67, 5902–5912 (2011)
Campo, V. L., Carvalho, I.: Click chemistry applied to carbohydrate-based drug discovery click chemistry in Glycoscience. New developments and strategies. In: Witczak, Z. J., Bielski, R., (eds); Wiley; Vol. 1, Chapter 13, pp 325–358 (2013)
Gouin S.G., Fernández J.M.G., Vanquelef E., Dupradeau F.-Y., Salomonsson E., Leffler H., Ortega-Muñoz M., Nilsson U.J., Kovensky J.: Multimeric lactoside “click clusters” as tools to investigate the effect of linker length in specific interactions with peanut lectin, galectin-1, and −3. Chembiochem. 11, 1430–1442 (2010)
Tejler J., Tullberg E., Frejd T., Leffler H., Nilsson U.J.: Synthesis of multivalent lactose derivatives by 1,3-dipolar cycloadditions: selective galectin-1 inhibition. Carbohydr. Res. 341, 1353–1362 (2006)
Salomonsson E., Larumbe A., Tejler J., Tullberg E., Rydberg H., Sundin A., Khabut A., Frejd T., Lobsanov Y.D., Rini J.M., Nilsson U.J., Leffler H.: Monovalent interactions of galectin-1. Biochemistry. 49, 9518–9532 (2010)
Mackeviča, J., Ostrovskis, P., Leffler, H., Nilsson, U. J., Rudovica, V., Viksna, A., Belyakov, S., Turks, M.: Synthesis of 1,2,3-triazole-linked galactohybrids and their inhibitory activities on galectins. ARKIVOC (iii) 90–112 (2014)
Glinsky G.V., Mossine V.V., Price J.E., Bielenberg D., Glinsky V.V., Ananthaswamy H.N., Feather M.S.: Inhibition of colony formation in agarose of metastatic human breast carcinoma and melanoma cells by synthetic glycoamine analogs. Clin. Exp. Metastasis. 14, 253–267 (1996)
Rabinovich G.A., Cumashi A., Bianco G.A., Ciavardelli D., Iurisci I., D’Egidio M., Piccolo E., Tinari N., Nifantiev N., Iacobelli S.: Synthetic lactulose amines: novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology. 16, 210–220 (2006)
Dominique R., Liu B., Das S.K., Roy R.: Synthesis of `molecular Asterisks' via sequential cross-metathesis, Sonogashira and Cyclotrimerization Reactions. Synthesis. 6, 862–868 (2000)
Roy R., Trono M.C., Giguère D.: Effects of linker rigidity and orientation of mannoside cluster for multivalent interactions with proteins. ACS Symp. Ser. 896, 137–150 (2005)
Roy R., Trono M.C., Giguère D.: Synthesis of stable and selective inhibitors of human galectins-1 and −3. Bioorg. Med. Chem. 16, 7811–7823 (2008)
Vrasidas I., André S., Valentini P., Böck C., Lensch M., Kaltner H., Liskamp R.M.J., Gabius H.-J., Pieters R.J.: Rigidified multivalent lactose molecules and their interactions with mammalian galectins: a route to selective inhibitors. Org. Biomol. Chem. 1, 803–810 (2003)
Bian C.-F., Zhang Y., Sun H., Li D.-F., Wang D.-C.: Structural basis for distinct binding properties of the human galectins to Thomsen-Friedenreich antigen. PLoS One. 6, (2011). doi:10.1371/journal.pone.0025007
Wang H., Huang W., Orwenyo J., Banerjee A., Vasta G.R., Wang L.-X.: Design and synthesis of glycoprotein-based multivalent glyco-ligands for influenza hemagglutinin and human galectin-3. Bioorg. Med. Chem. 21, 2037–2044 (2013)
Campo V.L., Riul T.B., Carvalho I., Baruffi M.D.: Antibodies against mucin-based Glycopeptides affect Trypanosoma cruzi cell invasion and tumor cell viability. Chembiochem. 15, 1495–1507 (2014)
Zhao Q., Barclay M., Hilkens J., Guo X., Barrow H., Rhodes J.M., Yu L.-G.: Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer. 9, (2010). doi:10.1186/1476–4598–9-154
André S., Ortega P.J.C., Perez M.A., Roy R., Gabius H.-J.: Lactose-containing starburst dendrimers: influence of dendrimer generation and binding-site orientation of receptors (plant/animal lectins and immunoglobulins) on binding properties. Glycobiology. 9, 1253–1261 (1999)
Michel A.K., Nangia-Makker P., Raz A., Cloninger M.J.: Lactose-functionalized dendrimers arbitrate the interaction of galectin-3/muc1 mediated cancer cellular aggregation. Chembiochem. 15, 2106–2112 (2014)
Böcker S., Laaf D., Elling L.: Galectin binding to neo-glycoproteins: LacDiNAc conjugated BSA as ligand for human galectin-3. Biomolecules. 5, 1671–1696 (2015)
Li J., Li W., Jinga J., Yua W.W.: Influencing factors on the synthesis of magnetically responsive lipases. J. Mol. Catal. B Enzym. 101, 47–55 (2014)
Rech C., Rosencrantz R.R., Křenek K., Pelantová H., Bojarová P., Römer C.E., Hanisch F.-G., Křen V., Elling L.: Combinatorial one-pot synthesis of poly-n-acetyllactosamine oligosaccharides with leloir-glycosyltransferases. Adv. Synth. Catal. 353, 2492–2500 (2011)
Takenaka Y., Fukumori T., Raz A.: Galectin-3 and metastasis. Glycoconj. J. 19, 543–549 (2004)
Ballell L., van Scherpenzeel M., Buchalova K., Liskamp R.M.J., Pieters R.J.: A new chemical probe for the detection of the cancer-linked galectin-3. Org. Biomol. Chem. 4, 4387–4394 (2006)
Acknowledgments
We acknowledge the financial support and fellowships from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo - Brazil) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Campo, V.L., Marchiori, M.F., Rodrigues, L.C. et al. Synthetic glycoconjugates inhibitors of tumor-related galectin-3: an update. Glycoconj J 33, 853–876 (2016). https://doi.org/10.1007/s10719-016-9721-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9721-z