Abstract
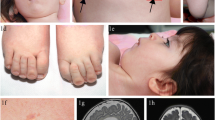
The human Golgi Cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-Sia) transporter SLC35A1, a member of the nucleotide sugar transporter family, translocates CMP-Sia from the cytosol into the Golgi lumen where sialyltransferases use it as donor substrate for the synthesis of sialoglycoconjugates. In 2005, we reported a novel Congenital Disorder of Glycosylation (CDG) termed CDG-IIf or SLC35A1-CDG, characterized by macrothrombocytopenia, neutropenia and complete lack of the sialyl-Lex antigen (NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAc-R) on polymorphonuclear cells. This disease was caused by the presence of inactive SLC35A1 alleles. It was also found that the SLC35A1 generates additional isoforms through alternative splicing. In this work, we demonstrate that one of the reported isoforms, the del177 with exon 6 skipping, is able to maintain sialylation in HepG2 cells submitted to wt knockdown and restore sialylation to normal levels in the Chinese Hamester Ovary (CHO) cell line Lec2 mutant deficient in CMP-Sia transport. The characteristics of the alternatively spliced protein are discussed as well as therapeutic implications of this finding in CDGs caused by mutations in nucleotide sugar transporters (NSTs).





Similar content being viewed by others
Abbreviations
- Ac4ManNAz:
-
N-azidoacetylmannosamine
- C/shRNA:
-
Control/shRNA plasmid
- CDG:
-
Congenital Disorder of Glycosylation
- CHO:
-
Chinese hamster ovary cell line
- CMP-Sia:
-
Cytidine-5′-monophospho-N-acetylneuraminic acid
- ER:
-
Endoplasmic Reticulum
- MO:
-
Morpholino
- MO-Scrambled:
-
Control morpholino oligo
- MO-Carboxy:
-
Carboxyfluorescein morpholino oligo
- MO-CMPTR6:
-
Morpholino directed against exon 6
- NST:
-
Nucleotide sugar transporter
- PCR:
-
Polymerase chain reaction
- PNA:
-
Peanut agglutinin
- RCA-I:
-
Ricinus communis agglutinin I
- SiaNAz:
-
N-azidoacetyl sialic acid
- SLC35A1/shRNA:
-
SLC35A1/shRNA plasmid
- WGA:
-
Wheat germ agglutinin
References
Martinez-Duncker I., Mollicone R., Codogno P., Oriol R.: The nucleotide-sugar transporter family: a phylogenetic approach. Biochimie. 85(3–4), 245–260 (2003)
Handford M., Rodriguez-Furlan C., Orellana A.: Nucleotide-sugar transporters: structure, function and roles in vivo. Braz. J. Med. Biol. Res. 39(9), 1149–1158 (2006)
Eckhardt M., Gotza B., Gerardy-Schahn R.: Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J. Biol. Chem. 273(32), 20189–20195 (1998)
Caffaro C.E., Hirschberg C.B.: Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc. Chem. Res. 39(11), 805–812 (2006)
Mironov, A. A.,Pavelka, M.: The Golgi apparatus: state of the art 110 years after Camillo Golgi’s discovery. Springer, Wien; New York (2008)
Furuichi T., Kayserili H., Hiraoka S., Nishimura G., Ohashi H., Alanay Y., Lerena J.C., Aslanger A.D., Koseki H., Cohn D.H., Superti-Furga A., Unger S., Ikegawa S.: Identification of loss-of-function mutations of SLC35D1 in patients with Schneckenbecken dysplasia, but not with other severe spondylodysplastic dysplasias group diseases. J. Med. Genet. 46(8), 562–568 (2009)
Hiraoka S., Furuichi T., Nishimura G., Shibata S., Yanagishita M., Rimoin D.L., Superti-Furga A., Nikkels P.G., Ogawa M., Katsuyama K., Toyoda H., Kinoshita-Toyoda A., Ishida N., Isono K., Sanai Y., Cohn D.H., Koseki H., Ikegawa S.: Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat. Med. 13(11), 1363–1367 (2007)
Mohamed M., Ashikov A., Guillard M., Robben J.H., Schmidt S., van den Heuvel B., de Brouwer A.P., Gerardy-Schahn R., Deen P.M., Wevers R.A., Lefeber D.J., Morava E.: Intellectual disability and bleeding diathesis due to deficient CMP–sialic acid transport. Neurology. 81(7), 681–687 (2013)
Jones C., Denecke J., Strater R., Stolting T., Schunicht Y., Zeuschner D., Klumperman J., Lefeber D.J., Spelten O., Zarbock A., Kelm S., Strenge K., Haslam S.M., Luhn K., Stahl D., Gentile L., Schreiter T., Hilgard P., Beck-Sickinger A.G., Marquardt T., Wild M.K.: A novel type of macrothrombocytopenia associated with a defect in alpha2,3-sialylation. Am. J. Pathol. 179(4), 1969–1977 (2011)
Willig T.B., Breton-Gorius J., Elbim C., Mignotte V., Kaplan C., Mollicone R., Pasquier C., Filipe A., Mielot F., Cartron J.P., Gougerot-Pocidalo M.A., Debili N., Guichard J., Dommergues J.P., Mohandas N., Tchernia G.: Macrothrombocytopenia with abnormal demarcation membranes in megakaryocytes and neutropenia with a complete lack of sialyl-Lewis-X antigen in leukocytes–a new syndrome? Blood. 97(3), 826–828 (2001)
Martinez-Duncker I., Dupre T., Piller V., Piller F., Candelier J.J., Trichet C., Tchernia G., Oriol R., Mollicone R.: Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood. 105(7), 2671–2676 (2005)
Stanley P., Siminovitch L.: Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 3(4), 391–405 (1977)
Riemersma M., Sandrock J., Boltje T.J., Bull C., Heise T., Ashikov A., Adema G.J., van Bokhoven H., Lefeber D.J.: Disease mutations in CMP-sialic acid transporter SLC35A1 result in abnormal alpha-dystroglycan O-mannosylation, independent from sialic acid. Hum. Mol. Genet. 24(8), 2241–2246 (2015)
Eckhardt M., Muhlenhoff M., Bethe A., Gerardy-Schahn R.: Expression cloning of the Golgi CMP-sialic acid transporter. Proc. Natl. Acad. Sci. U. S. A. 93(15), 7572–7576 (1996)
Ishida N., Ito M., Yoshioka S., Sun-Wada G.H., Kawakita M.: Functional expression of human golgi CMP-sialic acid transporter in the Golgi complex of a transporter-deficient Chinese hamster ovary cell mutant. J. Biochem. 124(1), 171–178 (1998)
Shin D.J., Kang J.Y., Kim Y.U., Yoon J.S., Choy H.E., Maeda Y., Kinoshita T., Hong Y.: Isolation of new CHO cell mutants defective in CMP-sialic acid biosynthesis and transport. Mol Cells. 22(3), 343–352 (2006)
Briles E.B., Li E., Kornfeld S.: Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J. Biol. Chem. 252(3), 1107–1116 (1977)
Parra M.K., Gee S., Mohandas N., Conboy J.G.: Efficient in vivo manipulation of alternative pre-mRNA splicing events using antisense morpholinos in mice. J. Biol. Chem. 286(8), 6033–6039 (2011)
Tyson-Capper A.J., Europe-Finner G.N.: Novel targeting of cyclooxygenase-2 (COX-2) pre-mRNA using antisense morpholino oligonucleotides directed to the 3′ acceptor and 5′ donor splice sites of exon 4: suppression of COX-2 activity in human amnion-derived WISH and myometrial cells. Mol. Pharmacol. 69(3), 796–804 (2006)
Chang P.V., Chen X., Smyrniotis C., Xenakis A., Hu T., Bertozzi C.R., Wu P.: Metabolic labeling of sialic acids in living animals with alkynyl sugars. Angew Chem Int Ed Engl. 48(22), 4030–4033 (2009)
Sharon N., Lis H.: Lectins, 2nd edn. Kluwer Academic Publishers, Boston (2003)
Patnaik S.K., Stanley P.: Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 416, 159–182 (2006)
Erber W.N., Asbahr H., Meyer B., Herrmann R.P., Davies J.M.: Peanut agglutinin (lectin from Arachis hypogaea) binding to hemopoietic cells: an immunophenotypic study using a biotin streptavidin technique. Pathology. 24(3), 173–176 (1992)
Aoki K., Ishida N., Kawakita M.: Substrate recognition by UDP-galactose and CMP-sialic acid transporters. Different sets of transmembrane helices are utilized for the specific recognition of UDP-galactose and CMP-sialic acid. J Biol Chem. 276(24), 21555–21561 (2001)
Takeshima-Futagami T., Sakaguchi M., Uehara E., Aoki K., Ishida N., Sanai Y., Sugahara Y., Kawakita M.: Amino acid residues important for CMP-sialic acid recognition by the CMP-sialic acid transporter: analysis of the substrate specificity of UDP-galactose/CMP-sialic acid transporter chimeras. Glycobiology. 22(12), 1731–1740 (2012)
Maggioni A., von Itzstein M., Rodriguez Guzman I.B., Ashikov A., Stephens A.S., Haselhorst T., Tiralongo J.: Characterisation of CMP-sialic acid transporter substrate recognition. Chembiochem. 14(15), 1936–1942 (2013)
Beitz E. (2000) T(E)Xtopo: shaded membrane protein topology plots in LAT(E)X2epsilon. Bioinformatics. 16(11):1050-1051
Zhao W., Chen T.L., Vertel B.M., Colley K.J.: The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. J. Biol. Chem. 281(41), 31106–31118 (2006)
Tusnady G.E., Simon I.: The HMMTOP transmembrane topology prediction server. Bioinformatics. 17(9), 849–850 (2001)
Eckhardt M, Gotza B, Gerardy-Schahn R.: Membrane topology of the mammalian CMP-sialic acid transporter. J Biol Chem. 274(13), 8779–87 (1999)
Quiroga R., Trenchi A., Gonzalez Montoro A., Valdez Taubas J., Maccioni H.J.: Short transmembrane domains with high-volume exoplasmic halves determine retention of type II membrane proteins in the Golgi complex. J. Cell Sci. 126(Pt 23), 5344–5349 (2013)
Pasquier C., Promponas V.J., Palaios G.A., Hamodrakas J.S., Hamodrakas S.J.: A novel method for predicting transmembrane segments in proteins based on a statistical analysis of the SwissProt database: the PRED-TMR algorithm. Protein Eng. 12(5), 381–385 (1999)
Chan K.F., Zhang P., Song Z.: Identification of essential amino acid residues in the hydrophilic loop regions of the CMP-sialic acid transporter and UDP-galactose transporter. Glycobiology. 20(6), 689–701 (2010)
Aoki K., Ishida N., Kawakita M.: Substrate recognition by nucleotide sugar transporters: further characterization of substrate recognition regions by analyses of UDP-galactose/CMP-sialic acid transporter chimeras and biochemical analysis of the substrate specificity of parental and chimeric transporters. J. Biol. Chem. 278(25), 22887–22893 (2003)
Oelmann S., Stanley P., Gerardy-Schahn R.: Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 276(28), 26291–26300 (2001)
Saini P., Gaur N.A., Prasad R.: Chimeras of the ABC drug transporter Cdr1p reveal functional indispensability of transmembrane domains and nucleotide-binding domains, but transmembrane segment 12 is replaceable with the corresponding homologous region of the non-drug transporter Cdr3p. Microbiology. 152(Pt 5), 1559–1573 (2006)
Lim S.F., Lee M.M., Zhang P., Song Z.: The Golgi CMP-sialic acid transporter: a new CHO mutant provides functional insights. Glycobiology. 18(11), 851–860 (2008)
Wang Q., Yin H., Camelliti P., Betts C., Moulton H., Lee H., Saleh A.F., Gait M.J., Wood M.J.: In vitro evaluation of novel antisense oligonucleotides is predictive of in vivo exon skipping activity for Duchenne muscular dystrophy. J Gene Med. 12(4), 354–364 (2010)
Acknowledgments
We are grateful to Dr. Alexandra Vincent from Gene Tools for her help in designing the antisense oligos morpholinos. We also acknowledge the support of the synthesis unit of the Instituto de Biotecnología-Universidad Nacional Autónoma de México in the synthesis of antisense oligos for PCR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
Work carried out in our laboratory was supported by CONACYT grant 57157. RSM was supported by CONACYT scholarship [217256]. IMD and RSM were supported by the Latin American Society for Glycobiology and the ECOS-CONACYT-SEP-ANUIES grant M09-S03. Red Temática Glicociencia en Salud- CONACYT grant 253596.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Salinas-Marín, R., Mollicone, R. & Martínez-Duncker, I. A functional splice variant of the human Golgi CMP-sialic acid transporter. Glycoconj J 33, 897–906 (2016). https://doi.org/10.1007/s10719-016-9697-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9697-8