Abstract
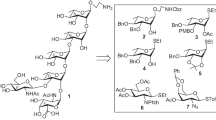
An acidic pentasaccharide repeating unit corresponding to the O-antigenic polysaccharide of enterohaemorrhagic Escherichia coli O113 as its p-methoxyphenyl glycoside has been synthesized in a convergent manner by adopting a [3+2] block glycosylation strategy. During the synthetic endeavor a one-pot reaction condition for stereoselective glycosylation and protecting group manipulation has been applied. All glycosylation steps are highly stereoselective with good to excellent yield.





Similar content being viewed by others
References
Karch, H., Tarr, P., Bielaszewska, M.: Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295, 405–418 (2005)
Russmann, H., Kothe, E., Schmidth, H., Franke, S., Harmsen, D., Caprioli, A., Karch, H.: Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J. Med. Microbiol. 42, 404–410 (1995)
Bower, J.R.: Food-borne diseases: Shiga toxin producing E. coli (STEC). Pediatr. J. Infect. Dis. 18, 909–910 (1999)
Ludwig, K., Sarkim, V., Bitzan, M., Karmali, M.A., Bobrowski, C., Ruder, H., Laufs, R., Sobottka, I., Petric, M., Karch, H., Muller-Wiefel, D.E.: Shiga toxin-producing Escherichia coli infection and antibodies against Stx2 and Stx1 in household contacts of children with enteropathic hemolytic-uremic syndrome. J. Clin. Microbiol. 40, 1773–1782 (2002)
Kaper, J.B.: Enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol. 1, 103–108 (1998)
Boyce, T.G., Swerdlow, D.L., Griffin, P.M.: Escherichia coli O157:H7 and the hemolytic uremic syndrome. N. Engl. J. Med. 333, 364–368 (1995)
Stenutz, R., Weintraub, A., Widmalm, G.: The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30, 382–403 (2006)
Roy, R.: New trends in carbohydrate based vaccines. Drug Discov. Today Technol. 1, 327–336 (2004). and references cited therein
Borman, S.A.: Carbohydrate vaccines: novel chemical and enzymatic oligosaccharide synthesis techniques could lead to a new generation of carbohydrate-based vaccine agents. Chem. Eng. News. 82, 31–35 (2004)
Doshi, G.M., Shanbhag, P.P., Aggarwal, G.V., Shahare, M.D., Martis, E.A.: Carbohydrate vaccines- A burgeoning field of Glycomics. J. Appl. Pharm. Sci. 1, 17–22 (2011)
Parolis, H., Parolis, L.A.S.: The structure of the O-specific polysaccharide from Escherichia coli O113 lipopolysaccharide. Carbohydr. Res. 267, 263–269 (1995)
Guchhait, G., Misra, A.K.: Convergent synthesis of a common pentasaccharide corresponding to the O-antigen of Escherichia coli O168 and Shigella dysenteriae type 4. Glyconjugate J. 28, 11–19 (2011)
Panchadhayee, R., Misra, A.K.: First synthesis of pentasaccharide repeating unit of the O-antigenic polysaccharide from enterohaemorrhagic Escherichia coli O48:H21. Tetrahedron: Asymm. 20, 1550–1555 (2009)
Kawai, Y., Ando, H., Ozeki, H., Koketsu, M., Ishihara, H.: A facile method for β-selenoglycoside synthesis using β-p-methylbenzoyl selenoglycoside as the selenating unit. Org. Lett. 7, 4653–4656 (2005)
Grundler, G., Schmidt, R.R.: Glycosyl imidates. 13. Application of the trichloroacetimidate procedure to 2-azidoglucose and 2-azidogalactose derivatives. Liebigs Annal. Chem. 11, 1826–1847 (1984)
Agnihotri, G., Tiwari, P., Misra, A.K.: Onepot synthesis of perOacetylated thioglycosides from unprotected reducing sugars. Carbohydr. Res. 340, 1393–1396 (2005)
Bhattacharyya, S., Magnusson, B.G., Wellmer, U., Nilsson, U.J.: The p-methoxybenzyl ether as an in situ-removable carbohydrate protecting group: a simple one-pot synthesis of the globotetraose tetrasaccharide. J. Chem. Soc. Perkin Trans. 1, 886–890 (2001)
Lemieux, R.U., Ratcliffe, R.M.: The azidonitration of tri-O-acetyl-D-galactal. Can. J. Chem. 57, 1244–1251 (1979)
Veeneman, G.H., van Leeuwen, S.H., van Boom, J.H.: Iodonium ion promoted reactions at the anomeric centre. II An efficient thioglycoside mediated approach toward the formation of 1,2-trans linked glycosides and glycosidic esters. Tetrahedron Lett. 31, 1331–1334 (1990)
Konradsson, P., Udodong, U.E., Fraser-Reid, B.: Iodonium promoted reactions of disarmed thioglycosides. Tetrahedron Lett. 31, 4313–4316 (1990)
Du, Y., Wei, G., Cheng, S., Hua, Y., Linhardt, R.J.: HClO4-SiO2 catalyzed glycosylation using trichloroacetimidates as glycosyl donors. Tetrahedron Lett. 47, 307–310 (2006)
Sau, A., Misra, A.K.: Environmentally benign preparation of benzylidene acetal of carbohydrate derivatives in PEG 600. J. Carbohydr. Chem. 30, 41–46 (2011)
Mukherjee, C., Misra, A.K.: Glycosylation and pyranose-furanose isomerization of carbohydrates using HClO4-SiO2: synthesis of oligosaccharides containing galactofuranose. Synthesis 683–692 (2007)
Agnihotri, G., Misra, A.K.: Mild and efficient method for the cleavage of benzylidene acetals using HClO4SiO2 and direct conversion of acetals to acetates. Tetrahedron Lett. 47, 3653–3658 (2006)
Werz, D.B., Seeberger, P.H.: Total Synthesis of Antigen Bacillus Anthracis Tetrasaccharide-Creation of an Anthrax Vaccine Candidate. Angew. Chem. Int. Ed. 44, 6315–6318 (2005)
Han, X.-B., Jiang, Z.-H., Schmidt, R.R.: Glycosyl Imidates, 61.–Synthesis of the Hexasaccharide Moiety of the Saponin Holotoxin A. Liebigs Annal. Chem. 853–858 (1993)
Bock, K., Lundt, I., Pedersen, C.: Assignment of anomeric structure to carbohydrates through geminal carbon-13-proton coupling constants. Tetrahedron Lett. 13, 1037–1040 (1973)
Crich, D., Li, H.: Synthesis of the Salmonella type E1 core trisaccharide as a probe for the generality of 1-(benzenesulfinyl)piperidine/triflic anhydride combination for glycosidic bond formation from thioglycoside. J. Org. Chem. 67, 4640–4646 (2002)
Misra, A.K., Ding, Y., Lowe, J.B., Hindsgaul, O.: A concise synthesis of the 6-O and 6′-O-sulfated analogues of the sialyl Lewis X tetrasaccharide. Bioorg. Med. Chem. Lett. 10, 1505–1509 (2000)
Huang, L., Teumelsan, N., Huang, X.: A facile method for oxidation of primary alcohols to carboxylic acids and its application in glycosaminoglycan syntheses. Chem. Eur. J. 12, 5246–5252 (2006)
Anelli, P.L., Biffi, C., Montanari, F., Quici, S.: Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 52, 2559–2562 (1987)
Panchadhayee, R., Misra, A.K.: Convergent synthesis of the pentasaccharide repeating unit of the O-antigen of Shigella boydii type 14. Tetrahedron: Asymm. 22, 1390–1394 (2011)
Chakraborti, A.K., Gulhane, R.: Perchloric acid adsorbed on silica gel as a new, highly efficient and versatile catalyst for acetylation of phenols, thiols, alcohols and amines. Chem. Commun. 1896–1897 (2003)
Acknowledgments
A. S. thanks CSIR, New Delhi for providing a Senior Research Fellowship. This work was supported by the Department of Science and Technology (DST), New Delhi (Project no. SR/S1/OC-83/2010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 4549 kb)
Rights and permissions
About this article
Cite this article
Santra, A., Misra, A.K. Convergent synthesis of the pentasaccharide repeating unit of the O-antigenic polysaccharide of enterohaemorrhagic Escherichia coli O113. Glycoconj J 29, 181–188 (2012). https://doi.org/10.1007/s10719-012-9383-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-012-9383-4