Abstract
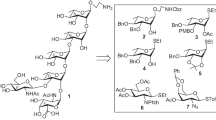
A convergent synthesis of the tetrasaccharide repeating unit of the O-antigen of the verotoxin producing E. coli O176 has been achieved in excellent yield adopting a [2 + 2] block glycosylation strategy. The β-D-mannosidic moiety of the tetrasaccharide was prepared from β-D-glucoside and α-D-galactosamine moiety was derived from D-galactal. The tetrasaccharide was synthesized as its 2-trimethylsilylethyl glycoside in excellent yield. All intermediate steps are high yielding.

A convergent synthetic strategy of the tetrasaccharide repeating unit corresponding to the O-antigen of verotoxin-producing Escherichia coli has been successfully developed using stereoselective [2 + 2] block glycosylation technique.



Similar content being viewed by others
References
Abbott, S.L., O’Conner, J., Robin, T., Zimmer, B.L., Janda, J.M.: Biochemical properties of a newly described Escherichia species, Escherichia albertii. J Clin Microbiol 41, 4852–4854 (2003)
Orskov, I., Orskov, F., Jann, B., Jann, K.: Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacterial Rev 41, 667–710 (1977)
Welinder-Olsson, C., Kaijser, B.: Enterohemorrhagic Escherichia coli (EHEC). Scand J Infect Dis 37, 405–416 (2005)
Karch, H., Tarr, P., Bielaszewska, M.: Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295, 405–418 (2005)
Ezawa, A., Gocho, F., Saitoh, M., Tamura, T., Kawata, K., Takahashi, T., Kikuchi, N.: A three year study of enterohemorrhagic Escherichia coli O157 on a farm in Japan. J. Vet. Med. Sc. 66, 779–784 (2004)
Boyce, T.G., Swerdlow, D.L., Griffin, P.M.: Escherichia coli O157:H7 and the hemolytic-Uremic Syndrome. N Engl J Med 1995(333), 364–368 (1995)
Stenutz, R., Weintraub, A., Widmalm, G.: The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev 30, 382–403 (2006)
Olsson, U., Weintraub, A., Widmalm, G.: Structural determination of the O-antigenic polysaccharide from the verocytotoxin-producing Escherichia coli O176. Carbohydr Res 343, 805–809 (2008)
Roy, R.: New trends in carbohydrate based vaccines. Drug Discov Today Tech 1, 327–336 (2004)
Doshi, G.M., Shanbhag, P.P., Aggarwal, G.V., Shahare, M.D., Martis, E.A.: Carbohydrate Vaccines- A burgeoning field of Glycomics. J Appl Pharm Sci 1, 17–22 (2011)
Jansson, K., Ahlfors, S., Frejd, T., Kihlberg, J., Magnusson, G.: 2-(Trimethylsilyl)ethyl glycosides. Synthesis, anomeric deblocking and transformation into 1,2-trans 1-O-acyl sugars. J. Org. Chem. 53, 5629–5647 (1988)
Mukhopadhyay, B., Collet, B., Field, R.A.: Glycosylation reactions with ‘disarmed’ thioglycoside donors promoted by N-iodosuccinimide and HClO4-silica. Tetrahedron Lett 46, 5923–5925 (2005)
Mukherjee, C., Misra, A. K.: Glycosylation and pyranose-furanose isomerization of carbohydrates using HClO4-SiO2: synthesis of oligosaccharides containing galactofuranose. Synthesis 683–692 (2007)
Chakraborti, A. K., Gulhane, R.: Perchloric acid adsorbed on silica gel as a new, highly efficient and versatile catalyst for acetylation of phenols, thiols, alcohols and amines. Chem. Commun. 1896–1897 (2003)
Kanie, O., Ito, Y., Ogawa, T.: Orthogonal glycosylation strategy in oligosaccharide synthesis. J Am Chem Soc 116, 12073–12074 (1994)
Misra, A.K., Roy, N.: Synthesis of a mannotetraose derivative related to the antigen from Escherichia coli O9a:K26:H-. Ind J Chem 36B, 308–311 (1997)
Schmidt, R.R., Grundler, G.: Glycosylimidates. Pt. 6. α-Bonded disaccharides from O-(β-D-glycopyranosyl) trichloroacetimidates with trimethylsilyltrifluoromethane sulfonate as catalyst. Angewandte Chemie 94, 790–791 (1982)
Sarkar, S.K., Mukhopadhyay, B., Roy, N.: Revised synthesis of pentasaccharide related to the repeating unit of the O-antigen from Shigella dysenteriae type 4 in the form of its methyl ester 2-(trimethylsilyl)ethyl glycoside. Ind J Chem 44B, 1058–1063 (2005)
Pearlman, W.M.: Noble metal hydroxides on carbon non pyrophoric dry catalysts. Tetrahedron Lett 8, 1663–1664 (1967)
Acknowledgements
G. G. thanks CSIR, New Delhi for providing a Senior Research fellowship. This project was supported by Bose Institute, Kolkata.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guchhait, G., Misra, A.K. Convergent synthesis of the tetrasaccharide repeating unit corresponding to the O-antigen of the verotoxin-producing Escherichia coli O176. Glycoconj J 28, 519–524 (2011). https://doi.org/10.1007/s10719-011-9351-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-011-9351-4