Abstract
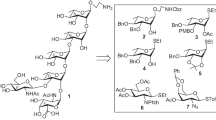
A convenient synthetic strategy of the common acidic pentasaccharide repeating unit corresponding to the O-antigen of enterotoxigenic E. coli O168 and Shigella dysenteriae type 4 has been successfully developed. A stereoselective [2 + 3] block glycosylation method has been exploited to get the target pentasaccharide derivative. Most of the synthetic intermediates were solid and prepared in high yields from commercially available reducing sugars following a series of protection-deprotection reactions. A α-D-mannose moiety has been used as the source of α-D-glucosamine moiety. A late-stage TEMPO mediated selective oxidation reaction finally resulted in the pentasaccharide containing a glucuronic acid unit.

A convenient synthetic strategy of the common acidic pentasaccharide repeating unit corresponding to the O-antigen of enterotoxigenic E. coli O168 and Shigella dysenteriae type 4 has been successfully developed using stereoselective [2+3] block glycosylation technique.





Similar content being viewed by others
References
Kaper, J.B., Nataro, J.P., Mobley, H.L.: Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004)
Nataro, J.P., Kaper, J.B.: Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201 (1998)
Kaper, J.B.: Enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol. 1, 103–108 (1998)
Ørskov, I., Ørskov, F., Jann, B., Jann, K.: Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41, 667–710 (1977)
Russmann, H., Kothe, E., Schmidth, H., Franke, S., Harmsen, D., Caprioli, A., Karch, H.: Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J. Med. Microbiol. 42, 404–410 (1995)
McConnell, M.M., Smith, H.R., Willshaw, G.A., Field, A.M., Rowe, B.: Plasmids coding for colonization factor antigen I and heat-stable enterotoxin production isolated from enterotoxigenic Escherichia coli: comparison of their properties. Infect. Immun. 32, 927–936 (1981)
Murray, B.E., Evans Jr., D.J., Penaranda, M.E., Evans, D.G.: CFA/I-ST plasmids: comparison of enterotoxigenic Escherichia coli (ETEC) of serogroups O25, O63, O78, and O128 and mobilization from an R factor-containing epidemic ETEC isolate. J. Bacteriol. 153, 566–570 (1983)
Wachsmuth, K., Morris, G.K.: Shigella. In: Doyle, M.P. (ed.) Foodborne bacterial pathogens, pp. 447–462. Marcel Dekker, New York (1989)
Chu, C., Liu, B., Watson, D., Szu, S., Bryla, D., Shiloach, J., Schneerson, R., Robbins, J.B.: Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect. Immun. 59, 4450–4458 (1991)
Dupont, H.L., Levine, M.M., Hornick, R.B., Formal, S.B.: Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159, 1126–1128 (1989)
Pupo, G.M., Lan, R., Reeves, P.R.: Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc. Natl Acad. Sci. USA 97, 10567–10572 (2000)
Hyma, K.E., Lacher, D.W., Nelson, A.M., Bumbaugh, A.C., Janda, J.M., Strockbine, N.A., Young, V.B., Whittam, T.S.: Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J. Bacteriol. 187, 619–628 (2005)
Perepelov, A.V., Liu, B., Senchenkova, S.N., Shashkov, A.S., Feng, L., Knirel, Y.A., Wang, L.: Close relation of the O-polysaccharide structure of Escherichia coli O168 and revised structure of the O-polysaccharide of Shigella dysenteriae type 4. Carbohydr. Res. 342, 2676–2681 (2007)
Dmitriev, B.A., L’vov, V.L., Kochetkov, N.K., Hofman, I.L.: Antigenic polysaccharides of bacteria. 6. The structure of O-specific polysaccharide chain of Shigella dysenteriae type 4 lipopolysaccharide. Bioorg. Khim. 3, 1226–1233 (1977)
Cohen, M.L.: Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257, 1050–1055 (1992)
Roy, R.: New trends in carbohydrate-based vaccines. Drug Discov Today Tech 1, 327–336 (2004). and references cited therein
Pozsgay, V.: Synthetic Shigella vaccines: a carbohydrate-protein conjugate with totally synthetic hexadecasaccharide haptens, Angew. Chem. Int. Ed. Engl. 37, 138–142 (1998)
Snippe, H., van Dam, J.E.G., van Houte, A.J., Willers, J.M.N., Kamerling, J.P., Vliegenthart, J.F.G.: Preparation of a semisynthetic vaccine to Streptococcus pneumoniae type 3. Infect. Immun. 42, 842–844 (1983)
Verez-Bencomo, V., Fernández-Santana, V., Hardy, E., Toledo, M.E., Rodríguez, M.C., Heynngnezz, L., Rodriguez, A., Baly, A., Herrera, L., Izquierdo, M., Villar, A., Valdés, Y., Cosme, K., Deler, M.L., Montane, M., Garcia, E., Ramos, A., Aguilar, A., Medina, E., Toraño, G., Sosa, I., Hernandez, I., Martínez, R., Muzachio, A., Carmenates, A., Costa, L., Cardoso, F., Campa, C., Diaz, M., Roy, R.: A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 305, 522–525 (2004)
Mukhopadhyay, B., Roy, N.: Synthesis of the pentasaccharide related to the repeating unit of the antigen from Shigella dysenteriae type 4 in the form of its methyl ester 2-(trimethylsilyl)ethyl glycoside. Carbohydr. Res. 338, 589–596 (2003)
Nakano, T., Ito, Y., Ogawa, T.: Total synthesis of a sulfated glucuronyl glycosphingolipid, IV3GlcA(3-SO3)nLcOse4Cer, a carbohydrate epitope of neural cell adhesion molecules. Tetrahedron Lett. 31, 1597–1600 (1990)
Depre, D., Duffels, A., Green, L.G., Lenz, R., Ley, S.V., Wong, C.H.: Synthesis of glycans from the Glycodelins: two undeca-, two deca-, three nona-, an octa- and a heptasaccharide. Chem. Eur. J. 5, 3326–3340 (1999)
van Steijn, A.M.P., Kamerling, J.P., Vliegenthart, J.F.G.: Synthesis of trisaccharide methyl glycosides related to fragments of the capsular polysaccharide of Streptococcus pneumoniae type 18C. Carbohydr. Res. 225, 229–246 (1992)
Madhusudan, S.K., Agnihotri, G., Negi, D.S., Misra, A.K.: Direct one-pot conversion of acylated carbohydrates into their alkylated derivatives under heterogeneous reaction conditions using solid NaOH and a phase transfer catalyst. Carbohydr. Res. 340, 1373–1377 (2005)
Veeneman, G.H., van Leeuwen, S.H., van Boom, J.H.: Iodonium ion promoted reactions at the anomeric centre. II An efficient thioglycoside mediated approach toward the formation of 1, 2-trans linked glycosides and glycosidic esters. Tetrahedron Lett. 31, 1331–1334 (1990)
Konradsson, P., Udodong, U.E., Fraser-Reid, B.: Iodonium promoted reactions of disarmed thioglycosides. Tetrahedron Lett. 31, 4313–4316 (1990)
Field, R.A., Otter, A., Fu, W., Hindsgaul, O.: Synthesis and 1H NMR characterization of the six isomeric mono-O-sulfates of 8-methoxycarbonyloct-1-yl O-β-D-galactopyranosyl-(1→4)-2-acetamido-2-deoxy-β-D-glucopyranoside. Carbohydr. Res. 276, 347–363 (1995)
Okiawa, Y., Yoshioko, T., Yonemitsu, O.: Specific removal of O-methoxybenzyl protection by DDQ oxidation. Tetrahedron Lett. 23, 885–888 (1982)
Pandey, S., Ghosh, S., Misra, A.K.: Synthesis of a trisaccharide and a tetrasaccharide from the cell-wall lipopolysaccharides of Azospirillum brasilense S17. Synthesis 2009, 2584–2590
Spijker, N.M., Keuning, C.A., Hooglugt, M., Veeneman, G.H., van Boeckel, C.A.A.: Synthesis of a hexasaccharide corresponding to a porcine zona pellucida fragment that inhibits porcine sperm-oocyte interaction in vitro. Tetrahedron 52, 5945–5960 (1996)
Misra, A.K., Ding, Y., Lowe, J.B., Hindsgaul, O.: A concise synthesis of the 6-O- and 6′-O-sulfated analogues of the sialyl Lewis X tetrasaccharide. Bioorg. Med. Chem. Lett. 10, 1505–1509 (2000)
Huang, L., Teumelsan, N., Huang, X.: A facile method for oxidation of primary alcohols to carboxylic acids and its application in glycosaminoglycan syntheses. Chem. Eur. J. 12, 5246–52 (2006)
Anelli, P.L., Biffi, C., Montanari, F., Quici, S.: Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 52, 2559–2562 (1987)
Davis, N.J., Flitsch, S.L.: Selective oxidation of monosaccharide derivatives to uronic acids. Tetrahedron Lett. 34, 1181–1184 (1993)
Acknowledgements
G. G. thanks CSIR, New Delhi for providing a Senior Research fellowship. This project was funded by the Department of Science and Technology (DST), New Delhi through Ramanna Fellowship (SR/S1/RFPC-06/2006) (A.K.M) and Bose Institute, Kolkata.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1060 kb)
Rights and permissions
About this article
Cite this article
Guchhait, G., Misra, A.K. Convergent synthesis of a common pentasaccharide corresponding to the O-antigen of Escherichia coli O168 and Shigella dysenteriae type 4. Glycoconj J 28, 11–19 (2011). https://doi.org/10.1007/s10719-010-9317-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-010-9317-y