Abstract
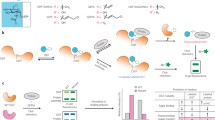
A substantial body of work has been devoted to the design and synthesis of glycosyltransferase inhibitors. A major obstacle has always been the demanding chemistry. Therefore, only few potent and selective inhibitors are known to date. Glycosyltransferases possess two distinct binding sites, one for the donor substrate, and one for the acceptor substrate. In many cases binding to the donor site is well defined but data for acceptor binding is sparse. In particular, acceptor binding sites are often shallow, and in many cases the dimensions of the binding pocket are not well defined. One approach to glycosyltransferase inhibitors is to chemically link donor site and acceptor site ligands to generate high affinity binders. Here, we describe a novel approach to identify acceptor site ligands from a fragment library. We have chosen human blood group B galactosyltransferase (GTB) as a biologically important model target. The approach utilizes a combination of STD NMR, spin-lock filtered NMR experiments and surface plasmon resonance measurements. Following this route we have identified molecular fragments from a fragment library that bind to the acceptor site of GTB with affinities of the order of a natural acceptor substrate. Unlike natural substrates these fragments allow for straightforward chemical modifications and, therefore will serve as scaffolds for potent GTB inhibitors. In general, the approach described is applicable to any glycosyltransferase and may assist in the development of novel glycosyltransferase inhibitors.





Similar content being viewed by others
References
Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E. (eds.): Essentials of glycobiology. Cold Spring Harbor Laboratory, New York (2009)
Brown, J.R., Fuster, M.M., Li, R., Varki, N., Glass, C.A., Esko, J.D.: A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin. Cancer Res. 12, 2894–2901 (2006)
Kannagi, R.: Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited. Glycoconj. J. 20, 353–364 (2004)
Kannagi, R., Izawa, M., Koike, T., Miyazaki, K., Kimura, N.: Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 95, 377–384 (2004)
Mathew, B., Schmidt, R.R.: Potential sialyltransferase inhibitors based on neuraminyl substitution by hetaryl rings. Carbohydr. Res. 342, 558–566 (2007)
Palcic, M.M., Heerze, L.D., Srivastava, O.P., Hindsgaul, O.: A bisubstrate analog inhibitor for alpha(1–2)-fucosyltransferase. J. Biol. Chem. 264, 17174–17181 (1989)
Jung, K.H., Schmidt, R.R.: Glycosyltransferase inhibitors. In: Wong, C.-H. (ed.) Carbohydrate-based drug discovery, pp. 609–659. Wiley-VCH, Weinheim (2003)
Hamilton, S.R., Gerngross, T.U.: Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr. Opin. Biotechnol. 18, 387–392 (2007)
Muller, B., Schaub, C., Schmidt, R.R.: Efficient sialyltransferase inhibitors based on transition-state analogues of the sialyl donor. Angew. Chem. Int. Edit. 37, 2893–2897 (1998)
Schaub, C., Muller, B., Schmidt, R.R.: New sialyltransferase inhibitors based on CMP-quinic acid: development of a new sialyltransferase assay. Glycoconj. J. 15, 345–354 (1998)
Skropeta, D., Schworer, R., Haag, T., Schmidt, R.R.: Asymmetric synthesis and affinity of potent sialyltransferase inhibitors based on transition-state analogues. Glycoconj. J. 21, 205–219 (2004)
Izumi, M., Yuasa, H., Hashimoto, H.: Bisubstrate analogues as glycosyltransferase inhibitors. Curr. Top Med. Chem. 9, 87–105 (2009)
Izumi, M., Kaneko, S., Yuasa, H., Hashimoto, H.: Synthesis of bisubstrate analogues targeting alpha-1, 3-fucosyltransferase and their activities. Org. Biomol. Chem. 4, 681–690 (2006)
Yazer, M.H., Olsson, M.L., Palcic, M.M.: The cis-AB blood group phenotype: fundamental lessons in glycobiology. Transfus. Med. Rev. 20, 207–217 (2006)
Alfaro, J.A., Zheng, R.B., Persson, M., Letts, J.A., Polakowski, R., Bai, Y., Borisova, S.N., Seto, N.O., Lowary, T.L., Palcic, M.M., Evans, S.V.: ABO(H) blood group A and B glycosyltransferases recognize substrate via specific conformational changes. J. Biol. Chem. 283, 10097–10108 (2008)
Patenaude, S.I., Seto, N.O., Borisova, S.N., Szpacenko, A., Marcus, S.L., Palcic, M.M., Evans, S.V.: The structural basis for specificity in human ABO(H) blood group biosynthesis. Nat. Struct. Biol. 9, 685–690 (2002)
Mayer, M., Meyer, B.: Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. 38, 1784–1788 (1999)
Scherf, T., Anglister, J.: A T1 rho-filtered two-dimensional transferred NOE spectrum for studying antibody interactions with peptide antigens. Biophys. J. 64, 754–761 (1993)
Jahnke, W., Rudisser, S., Zurini, M.: Spin label enhanced NMR screening. J. Am. Chem. Soc. 123, 3149–3150 (2001)
Meyer, B., Peters, T.: NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors. Angew. Chem. Int. Ed. Engl. 42, 864–890 (2003)
Ludwig, C., Guenther, U.L.: Ligand based NMR methods for drug discovery. Front. Biosci. 14, 4565–4574 (2009)
Diercks, T., Coles, M., Kessler, H.: Applications of NMR in drug discovery. Curr. Opin. Chem. Biol. 5, 285–291 (2001)
Coles, M., Heller, M., Kessler, H.: NMR-based screening technologies. Drug. Discov. Today 8, 803–810 (2003)
Pellecchia, M., Bertini, I., Cowburn, D., Dalvit, C., Giralt, E., Jahnke, W., James, T.L., Homans, S.W., Kessler, H., Luchinat, C., Meyer, B., Oschkinat, H., Peng, J., Schwalbe, H., Siegal, G.: Perspectives on NMR in drug discovery: a technique comes of age. Nat. Rev. Drug Discov. 7, 738–745 (2008)
Wyss, D.F., McCoy, M.A., Senior, M.M.: NMR-based approaches for lead discovery. Curr. Opin. Drug Discov. Devel. 5, 630–647 (2002)
Marcus, S.L., Polakowski, R., Seto, N.O., Leinala, E., Borisova, S., Blancher, A., Roubinet, F., Evans, S.V., Palcic, M.M.: A single point mutation reverses the donor specificity of human blood group B-synthesizing galactosyltransferase. J. Biol. Chem. 278, 12403–12405 (2003)
Hwang, T.L., Shaka, A.J.: Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. A 112, 275–279 (1995)
Sindhuwinata, N., Miunoz, E., Munoz, F.J., Palcic, M.M., Peters, H., Peters, T.: Binding of an acceptor substrate analog enhances the enzymatic activity of human blood group B galactosyltransferase. Glycobiology. in press.
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., Olson, A.J.: AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. (2009)
Blume, A., Angulo, J., Biet, T., Peters, H., Benie, A.J., Palcic, M., Peters, T.: Fragment-based screening of the donor substrate specificity of human blood group B galactosyltransferase using saturation transfer difference NMR. J. Biol. Chem. 281, 32728–32740 (2006)
Shoemaker, G.K., Soya, N., Palcic, M.M., Klassen, J.S.: Temperature-dependent cooperativity in donor-acceptor substrate binding to the human blood group glycosyltransferases. Glycobiology 18, 587–592 (2008)
Stockman, B.J., Dalvit, C.: NMR screening techniques in drug discovery and drug design. Prog. Nucl. Magn. Reson. Spectrosc. 41, 187–231 (2002)
Dalvit, C., Flocco, M., Knapp, S., Mostardini, M., Perego, R., Stockman, B.J., Veronesi, M., Varasi, M.: High-throughput NMR-based screening with competition binding experiments. J. Am. Chem. Soc. 124, 7702–7709 (2002)
Manzenrieder, F., Frank, A.O., Kessler, H.: Phosphorus NMR spectroscopy as a versatile tool for compound library screening. Angew. Chem. Int. Ed. Engl. 47, 2608–2611 (2008)
Klages, J., Coles, M., Kessler, H.: NMR-based screening: a powerful tool in fragment-based drug discovery. Analyst 132, 693–705 (2007)
Leone, M., Freeze, H.H., Chan, C.S., Pellecchia, M.: The Nuclear overhauser effect in the lead identification process. Curr. Drug. Discov. Technol. 3, 91–100 (2006)
Sem, D.S., Pellecchia, M.: NMR in the acceleration of drug discovery. Curr. Opin. Drug Discov. Devel. 4, 479–492 (2001)
Schuffenhauer, A., Ruedisser, S., Marzinzik, A.L., Jahnke, W., Blommers, M., Selzer, P., Jacoby, E.: Library design for fragment based screening. Curr. Top Med. Chem. 5, 751–762 (2005)
Rudisser, S., Jahnke, W.: NMR and in silico screening. Comb. Chem. High Throughput Screen 5, 591–603 (2002)
Acknowledgment
This work was supported by grants from the Swedish Research Council (VR), The Knut and Alice Wallenberg Foundation and Magn. Bergvalls Stiftelse (G.W.). J.L. acknowledges the Deutscher Akademischer Austausch Dienst and the Swedish Institute for financial support. T.P. acknowledges grants the German Research Council (DFG, HBFG 101/192-1 and ME 1830/1), from the state of Schleswig-Holstein (Innovationsfonds 2005), and from the University of Lübeck. C.R. thanks the Fonds der Chemischen Industrie for a stipend. N.S. thanks the Studienstiftung des Deutschen Volkes for a stipend.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting information available
Structures of the Maybridge Ro5 library and all experimental data from the screening are available.
This paper is dedicated to Prof. Klaus Bock on the occasion of his 65th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 27 kb)
Rights and permissions
About this article
Cite this article
Rademacher, C., Landström, J., Sindhuwinata, N. et al. NMR-based exploration of the acceptor binding site of human blood group B galactosyltransferase with molecular fragments. Glycoconj J 27, 349–358 (2010). https://doi.org/10.1007/s10719-010-9282-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-010-9282-5