Abstract
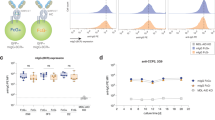
Little is known about the glycosylation of the isotype switched B cell receptor (BCR) in multiple myeloma, and the way it might affect receptor function. In this work IgG BCRs isolated from the individual lysates of peripheral blood lymphocytes (PBL) of 32 patients with IgG multiple myeloma and healthy controls were investigated for the expression of sialic acid (SA), galactose (Gal) and N-acetylglucosamine (GlcNAc), the sugars known to specify the glycoforms of human serum IgG. The degree of glycosylation and signaling status of all 32 isolated myeloma IgG BCRs were correlated and compared with the glycosylation of the IgG paraproteins isolated from sera of the same patients. It was shown that BCR IgG in myeloma is more heavily sialylated when compared with normal controls, that the increased sialylation of IgG BCR is associated with higher levels of tyrosine phosphorylation (signaling activity) of the IgG BCR supramolecular complex and that BCR IgG and serum IgG paraprotein from the same patient differed in all cases in the levels of terminal sugar expression. The results suggest that the development of the malignant clone in MM from post-switch B cells expressing IgG BCR at their surfaces to plasma cells secreting IgG paraprotein may be followed by permanent glycosylation changes in the IgG molecules.







Similar content being viewed by others
References
Bakkus, M.H., van Riet, I., de Graff, C., van Camp, B., Thielemans, K.: The clonogenic precursor cell in multiple myeloma. Leuk. Lymphoma 18, 221–229 (1995)
Bradford, M.A.: A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72, 248–254 (1976)
Cohen, A.M., Allalouf, D., Bessler, H., Djaldetti, M., Malachi, T., Levinsky, H.: Sialyltransferase activity and sialic acid levels in multiple myeloma and monoclonal gammopathy. Eur. J. Immunol 42, 289–292 (1989a)
Cohen, A.M., Allalouf, D., Djaldetti, M., Weigl, K., Lehrer, N., Levinsky, H.: Sialyltransferase activity in plasma cells of multiple myeloma. Eur. J. Immunol 43, 191–194 (1989b)
Cordoba, F., Lavabre-Bertrand, T., Salhi, ShL., Huguet, M.F., Gerfaux, J., Rossi, J.F., Vendrell, J.P.: Spontaneous monoclonal immunoglobulin-secreting peripheral blood mononuclear cells as a marker of disease severity in multiple myeloma. Br. J. Haematol 108, 549–558 (2000)
Cyster, J.G., Goodnow, ChC.: Tuning antigen receptor signaling by CD22: Integrating cues from antigens and microenvironment. Immunity 6, 509–517 (1997)
Davidson, H.W., West, M.A., Watts, C.: Endocytosis, intracellular trafficking, and processing of membrane IgG and monovalent antigen/membrane IgG complexes in B lymphocytes. J. Immunol 144, 4101–4109 (1990)
Doody, G.M., Justement, L.B., Delibrias, C.C., Matthews, R.J., Lin, J., Thomas, M.L., Fearon, D.T.: A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269, 242–244 (1995)
Endo, T., Wright, A., Morrison, S.L., Kobata, A.: Glycosylation of the variable region of immunoglobulin G—site specific maturation of the sugar chains. Mol. Immunol 32, 931–940 (1995)
Erikstein, B.K., Funderud, S., Beiske, K., Aas-Eng, A., De Lange Davies, C., Blomhoff, H.K., Smeland, E.B.: Cell cycle-dependent regulation of CDw75 (beta-galactoside alpha-2,6-sialyltransferase) on human B lymphocytes. Eur. J. Immunol 22, 1149–1155 (1992)
Farooq, M., Takahashi, N., Arrol, H., Drayson, M., Jefferis, R.: Glycosylation of polyclonal and paraprotein IgG in multiple myeloma. Glycoconj. J 14, 489–492 (1997)
Farooq, M., Takahashi, N., Drayson, M., Lund, J., Jefferis, R.: A longitudinal study of glycosylation of human IgG3 paraprotein in patient with multiple myeloma. Glycoimmunol 2, 95–103 (1998)
Fleming, S.C., Smith, S., Knowles, D., Skillen, A., Self, C.H.: Increased sialylation of oligosaccharides on IgG paraproteins—a potential new tumour marker in multiple myeloma. J. Clin. Pathol 51, 825–830 (1998)
Frithz, G., Ronquist, G., Ericsson, P.: Serum sialyltransferase and fucosyltransferase activities in patients with multiple myeloma. Eur. J. Cancer Clin. Oncol 21, 913–917 (1985)
Ghosh, S., Bandulet, C., Nitschke, L.: Regulation of B cell development and B cell signaling by CD22 and its ligands α2,6-linked sialic acids. Int. Immunol 18, 603–611 (2006)
Horikawa, K., Martin, S.W., Pogue, S.L., Siver, K., Peng, K., Takatsu, K., Goodnow, ChC.: Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J. Exp. Med 204, 758–69 (2007)
Ilić, V., Milošević-Jovčić, N., Petrović, S., Marković, D., Bila, J., Bošković, D., Stefanović, G., Marković, O., Glibetić, M.: Signaling status of IgG B cell receptor (IgG BCR) is indicative for an activated state of circulating B cells in multiple myeloma. Ann. Hematol 86, 905–912 (2007)
Jefferis, R., Lund, J., Mizutani, H., Takagawa, H., Kawazoe, Y., Arata, Y., Takahashi, N.: A comparative study of the N-linked oligosaccharide structures of human IgG subclass proteins. Biochem. J 268, 529–537 (1990)
Jefferis, R., Lund, J., Puond, J.D.: IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol. Rev 163, 59–76 (1998)
Jin, L., McLean, P.A., Neel, B.G., Wortis, H.H.: Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J. Exp. Med 195, 1199–1205 (2002)
Kaneko, Y., Nimmerjahn, F., Ravetch, J.V.: Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006)
Lankester, A.C., van Schijndel, G.M.W., Frommé, J., Cordell, J.L., van Lier, R.A.W.: van Noesel CJM, Evidence for a direct physical interaction of membrane IgM, IgD, and IgG with B29 gene product. J. Immunol 152, 2157–2162 (1994)
Leibiger, H., Wustner, D., Stigler, R.D., Marx, U.: Variable domain-linked oligosaccharides of a human monoclonal IgG: structure and influence on antigen binding. Biochem. J 338, 529–538 (1999)
Martin, S.W.: Goodnow ChC, Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol 3, 182–188 (2002)
McCann, K.J., Johnson, P.W.M., Stevenson, F.K., Ottensmeier, ChH.: Universal N-glycosylation sites introduced into the B-cell receptor of follicular lymphoma by somatic mutation: a second tumorigenic event? Leukemia 20, 530–534 (2006)
Mimura, Y., Ashton, P.R., Takahashi, N., Harvey, D.J., Jefferis, R.: Contrasting glycosylation profiles between Fab and Fc of a human IgG protein studied by electrospray ionization mass spectrometry. J. Immunol. Methods 326, 116–126 DOI 10.1016/j.jim.2007.07.014 (2007)
Minuzzo, S., Indraccolo, S., Tosello, V., Piovan, E., Cabrelle, A., Trentin, L., Semenzato, G., Amadori, A.: Heterogenous intracellular expression of B-cell receptor components in B-cell chronic lymphocytic leukaemia (B-CLL) cells and effects of CD79b gene transfer on surface immunoglobulin levels in a B-CLL-derived cell line. Br. J. Haematol 130, 878–889 (2005)
Mizuochi, T., Taniguchi, T., Shimizu, A., Kobata, A.: Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J. Immunol 129, 2016–2020 (1982)
Monroe, J.G.: Antigen receptor-initiated signals for B cell development and selection. Immunol. Res 17, 155–162 (1998)
Niiro, H., Clark, E.A.: Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol 2, 945–956 (2002)
Nishiura, T., Fujii, S., Kanayama, Y., Nishikawa, A., Tomiyama, Y., Iida, M., Karasuno, T., Nakao, H., Yonezawa, T., Taniguchi, N., Tarui, S.: Carbohydrate analysis of immunoglobulin G myeloma proteins by lectin and high performance liquid chromatography: role of glycosyltransferases in the structures. Cancer Res 50, 5345–5350 (1990)
Ota, T., Higashi, S., Suzuki, H., Eto, S.: Enzyme linked immunosorbent assay (ELISA) for human IgG (Gm) allotype determination. Mol. Immunol 28, 51–56 (1991)
Perfetti, V., Vignarelli, M.C., Bellotti, V., Glennie, M.J., Yoryoli, I., Ubbiali, P., Obici, L., Massa, M., Ippoliti, G., Ascari, E., Merlini, G.: Membrane CD22 defines circulating myeloma-related cells as mature or later B cells. Lab. Invest 77, 333–344 (1997)
Pilarski, L.M., Masellius-Smith, A., Szczepek, A., Mant, M.J., Belch, A.R.: Circulating clonotypic B cells in the biology of multiple myeloma: speculations on the origin of myeloma. Leuk. Lymphoma 22, 375–383 (1996)
Radcliffe, C.M., Arnold, J.N., Suter, D.M., Wormald, M.R., Harvey, D.J., Royle, L., Mimura, Y., Kimura, Y., Sim, R.B., Inogès, S., Rodrigez-Calvillo, M., Zabalegui, N., López-Díaz de Cerio, A., Potter, K.N., Mocridge, C.I., Dwek, R.A.: Bendandi M, Rudd PM, Stevenson FK, Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B cell receptor. J. Biol. Chem 282, 7405–7415 (2007)
Radl, J., Wells, J., Hoogeveen, C.M.: Immunoblotting with (sub)class specific antibodies reveales a high frequency of monoclonal gammapathies in persons thought to be immunodeficient. Clin. Chem 34, 1839–1842 (1988)
Santini, L.Q., Rosenspire, A.R.: Glycosylated extracellular domains of membrane immunoglobulin M contribute to its association with mb-1/B29 gene products and the B cell receptor complex. Immunol. Invest 27, 57–72 (1998)
Stevenson, G.T., Kan, K.S.: Chemically engineered antibodies. In: Johnstone, A.P., Turner, M.W. (eds.) Immunochemistry 1, a practical approach, pp. 1–25. IRL Press at Oxford University Press, Oxford (1997)
Sumar, N., Bodman, K.B., Rademacher, T.W., Dwek, R.A., Williams, P., Parekh, R.B., Edge, J., Rook, G.A.W., Isenberg, D.A., Hay, F.C., Roitt, I.M.: Analysis of glycosylation changes in IgG using lectins. J. Immunol. Methods 131, 127–136 (1990)
Takahashi, N., Ishii, I.: Comparative structural studies of N-linked oligosaccharides of human and pathological immunoglobulin G. Biochemistry 26, 1137–1144 (1987)
Vullier, F., Dumas, G., Magnac, Ch., Prevost, M-Ch., Lalanne, A-I., Oppezzo, P., Melanitou, E., Dighiero, G., Payelle-Brogard, B.: Lower levels of surface B-cell receptor expression in chronic lymphocytic leukemia are associated with glycosylation and folding defects of the μ and CD79a chains. Blood 105, 2933–2940 (2005)
Wagle, N.M., Kim, J.H., Pierce, S.K.: Signaling through the B cell antigen receptor regulates discrete steps in the antigen processing pathway. Cell. Immunol 184, 1–11 (1998)
Wakabayashi, Ch., Adachi, T., Wienends, J., Tsubata, T.: A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 298, 2392–2395 (2002)
Weisman, A., Kraus, M., Seagal, J., Gnosh, S., Melamed, D., Song, J., Sasaki, Y., Classen, S., Luty, C., Brombachert, F., Nitschke, L., Rajewski, K.: IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Igα/β. J. Exp. Med 204, 747–758 (2007)
Zabalegui, N., de Cerio, A.L., Inoges, S., Rodriguez-Calvillo, M., Perez-Calvo, J., Hernandez, M., Garcia-Foncillas, J., Martin-Algarra, S., Rocha, E., Bendandi, M.: Acquired potential N-glycosylation sites within the tumor-specific immunoglobulin heavy chains of B-cell malignancies. Haematologica 89, 541–546 (2004)
Zhu, D., McCarthy, H., Ottensmeier, ChH., Johnson, P., Hamblin, T.J., Stevenson, F.K.: Acquisition of potential N-glycosylation sites in immunoglobulin variable region by somatic mutation is distinctive feature of follicular lymphoma. Blood 99, 2562–2568 (2002)
Acknowledgements
The Ministry of Science of Serbia supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilić, V., Milošević-Jovčić, N., Petrović, S. et al. Glycosylation of IgG B cell receptor (IgG BCR) in multiple myeloma: relationship between sialylation and the signal activity of IgG BCR. Glycoconj J 25, 383–392 (2008). https://doi.org/10.1007/s10719-007-9101-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-007-9101-9