Abstract
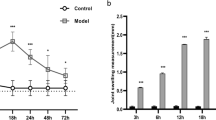
Treatment of macrophages with various doses of wheat germ agglutinin (WGA) for different time intervals resulted in enhanced expression of TNF-α, IL-1β, IL-12 and IFN-γ. The maximum expressions were observed at 24 h with 100 ng/ml of WGA. Enhanced transcription of cytokines TNF-α, IL-1β, IL-12, and IFN-γ was observed at 16 h of WGA treatment by RT-PCR. Pharmacological inhibitor of tyrosine kinase, PI3 kinase, protein kinase C, p42/44, p38, JNK and intracellular calcium immobilizing agent down regulated the WGA induced expression of cytokines TNF-α, IL-1β, IL-12 and IFN-γ. Maximum protein tyrosine kinase activity in macrophages was seen at 5 min of WGA treatment. Maximum cytosolic Ca++ was observed at 10 min of WGA treatment. WGA treated macrophages showed maximum activation of protein kinase C (PKC) and PI3 kinase at 10 min, p42/44, p38 at 15 min and JNK at 30 min. Transcription factor ELK1 was activated at 60 min and IêB, c-Fos and c-Jun at 30 min of WGA treatment. The pharmacological inhibitors were also used to check the cascade of activation of tyrosine kinase, PKC, PI3 kinase, p42/44, p38, JNK and release of calcium from intracellular storage to sort out the signal pathways involved in the release of TNF-α, IL-1β, IL-12, and IFN-γ by macrophages on treatment with WGA in vitro.









Similar content being viewed by others
References
Rosen, S.D., Bertozzi, C.R.: The selectins and their ligands. Curr. Opin. Cell. Biol. 6, 663–673 (1994)
Ofek, I., Sharon, N.: Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect. Immun. 56, 539–547 (1988)
Drysdale, B.E., Yapundich, R.A., Shin, M.L., Shin, H.S.: Lipopolysaccharide-mediated macrophage activation: the role of calcium in the generation of tumoricidal activity. J. Immunol. 138, 951–956 (1987)
Oppenheim, J.J., Rosenstreich, D.L.: Signals regulating in vitro activation of lymphocytes. Prog. Allergy. 20, 65–194 (1976)
Perez, H.D., Elfman, F., Lobo, E.: Removal of human polymorphonuclear leukocyte surface sialic acid inhibits reexpression (or recycling) of formyl peptide receptors. A possible explanation for its effect on formyl peptide-induced polymorphonuclear leukocyte chemotaxis. J. Immunol. 139, 1978–1984 (1987)
Sharon, N., Lis, H.: Lectins: cell-agglutinating and sugar-specific proteins. Science 177, 949–959 (1972)
Cohen, M.S., Metcalf, J.A., Root, R.K.: Regulation of oxygen metabolism in human granulocytes: relationship between stimulus binding and oxidative response using plant lectins as probes. Blood 55, 1003–1010 (1980)
Hartshorn, K.L., Daigneault, D.E., White, M.R., Tauber, A.I.: Anomalous features of human neutrophil activation by influenza A virus are shared by related virus and sialic acid-binding lectins. J. Leukoc. Biol. 51, 230–236 (1992)
Lock, R., Johansson, A., Orselius, K., Dahlgren, C.: Analysis of horseradish peroxidase amplified chemiluminescence produced by human neutrophils reveals a role for the superoxide anion in the light emitting reaction. Anal. Biochem. 173, 450–455 (1988)
Magnusson, K.E., Dahlgren, C., Sjolander, A.: Distinct pattern of granulocyte luminol-dependent chemiluminescence response to lectins WGA and RCA-1. Inflammation 12, 17–24 (1988)
Keisari, Y., Braun, L., Flescher, E.: The oxidative burst and related phenomena in mouse macrophages elicited by different sterile inflammatory stimuli. Immunobiology 165, 78–89 (1983)
Tomioka, H., Saito, H.: Comparison of wheat germ agglutinin-and phorbol myristate acetate-mediated triggering for macrophage H2O2 release: susceptibilities to various macrophage inhibitors. Microbiol. Immunol. 31, 211–221 (1987)
Karlsson, A.: Wheat germ agglutinin induces NADPH-oxidase activity in human neutrophils by interaction with mobilizable receptors. Infect. Immun. 67, 3461–3468 (1999)
Stoika, R., Kashchak, N., Lutsik-Kordovsky, M., Boyko, M., Tsyrulnyk, A.: In vitro response of phagocytic cells to immunomodulating agents. Med. Sci. Monit. 7, 652–658 (2001)
Chattopadhyay, U., Bhattacharyya, S.: Induction of tumor associated macrophage-mediated lysis of autologous tumor cells by lectins. Neoplasma 35, 321–328 (1988)
Kesherwani, V., Sodhi, A.: Differential activation of macrophages in vitro by lectin Concanavalin, A., Phytohemagglutinin and Wheat germ agglutinin: Production and Regulation of Nitric oxide. Nitric Oxide 16, 294–305 (2007)
Adams, D.O., Nathan, C.F.: Molecular mechanisms in tumor-cell killing by activated macrophages. Immunol. Today 4, 166–170 (1983)
Key, M.E., Hoyer, L., Bucana, C., Hanna, M.G. Jr.: Mechnaism of macrophage mediated tumor cytolysis. Adv. Exp. Med. Biol. 146, 265–310 (1982)
Klostergaard, J., Leroux, M.E., Hung, M.C.: Cellular models of macrophage tumoricidal effector mechanism in vitro. Characterization of cytolytic response to tumor necrosis factor and nitric oxide pathways in vitro. J. Immunol. 147, 2802–2808 (1991)
Sodhi, A., Singh, S.M.: Release of cytolytic factor(s) by murine macrophages in vitro on treatment with cis-platin. Int. J. Immunopharmacol. 8, 701–707 (1986)
Ranjan, P., Sodhi, A., Srivastava, A.: Cispaltin and interferon-gamma treated murine macrophage induce apoptosis in tumor cell lines. Anticancer Drugs 8, 1–10 (1997)
Hibbs, J.B. Jr., Taintor, R.R., Vavrin, Z., Rachlin, E.M.: Nitric Oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157, 87–94 (1988)
Joo, S.S., Chang, J.K., Park, J.H., Kang, H.C., Lee, D.I.: Immunoactivation of lectin-conjugated praecoxin A on IL-6, IL-12 expression. Arch. Pharm. Res. 25, 954–963 (2002)
Guan, J.L., Trevithick, J.E., Hynes, R.O.: Fibronectin/integrin interaction induces tyrosine phosphorylation of 120Kda proteins. Cell. Regul. 2, 951–964 (1991)
Cooper, G.M.: The Cell a Molecular Approach, pp. 521–559. ASM, Washington, DC (2000)
Sodhi, A., Sethi, G.: Involvement of MAP kinase signal transduction pathway in UVB-induced activation of macrophages in vitro. Immunol. Lett. 90, 123–130 (2003)
Watowich, S.S., Wu, H., Socolovsky, M., Klingmuller, U., Constantinescu, S.N., Lodish, H.F.: Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu. Rev. Cell. Dev. Biol. 12, 91–128 (1996)
Inazu, T., Taniguchi, T., Ohta, S., Miyabo, S., Yamamura, H.: The lectin wheat germ agglutinin induces rapid protein-tyrosine phosphorylation in human platelets. Biochem. Biophys. Res. Commun. 174, 1154–1158 (1991)
Ohmori, T., Yatomi, Y., Wu, Y., Osada, M., Satoh, K., Ozaki, Y.: Wheat germ agglutinin-induced platelet activation via platelet endothelial cell adhesion molecule-1: involvement of rapid phospholipase C gamma 2 activation by Src family kinases. Biochemistry 40, 12992–13001 (2001)
Sodhi, A., Singh, R.K., Singh, S.M.: Effect of interferon-gamma priming on the activation of murine peritoneal macrophages to the tumouricidal state by cisplatin, IL-1, and tumour necrosis factor (TNF): production of IL-1 and TNF. Clin. Exp. Immunol. 88, 350–355 (1992)
Sethi, G., Sodhi, A.: In vitro activation of murine peritoneal macrophages by ultraviolet B radiation: upregulation of CD18, production of NO, proinflammatory cytokines and a signal transduction pathway. Mol. Immunol. 40, 1315–1323 (2004)
Sodhi, A., Sethi, G.: Involvement of MAP kinase signal transduction pathway in UVB-induced activation of macrophages in vitro. Immunol. Lett. 90, 123–130 (2003)
Singh, R.A., Sodhi, A.: Cisplatin-treated macrophages produce oncostatin M: regulation by serine/threonine and protein tyrosine kinases/phosphatases and Ca2+/calmodulin. Immunol. Lett. 62, 159–164 (1998)
Mosmann, T.: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983)
Biswas, S.K., Sodhi, A.: In vitro activation of murine peritoneal macrophages by monocyte chemoattractant protein-1: upregulation of CD11b, production of proinflammatory cytokines, and the signal transduction pathway. J. Interferon. Cytokine. Res. 5, 527–538 (2002)
Hamilton, T.A., Adams, D.O.: Molecular mechanisms of signal transduction in macrophages. Immunol. Today 8, 151–158 (1987)
Weinstein, S.L., Gold, M.R., DeFranco, A.L.: Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc. Natl. Acad. Sci. U. S. A. 88, 4148–4152 (1991)
Kumar, R., Sodhi, A.: Activation of murine peritoneal macrophages with cisplatin and lipopolysaccharide: involvement of protein kinase C and tyrosine kinase. J. Clin. Biochem. Nutr. 17, 53–64 (1994)
Celada, A., Schreiber, R.D.: Role of protein kinase C and intracellular calcium mobilization in the induction of macrophages tumoricidal activity by Interferon-gamma. J. Immunol. 137, 2373–2379 (1986)
Nakane, M., Mitchell, J., Förstermann, U., Murad, F.: Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem. Biophys. Res. Commun. 180, 1396–1402 (1991)
Eason, S., Martin, W.: Involvement of tyrosine kinase and protein kinase C in the induction of nitric oxide synthase by lipopolysaccharids and interferon-gamma in J774 macrophages. Arch. Int. Pharmacodyn. 330, 225–236 (1995)
Friedman, B., Frackelton, A.R. Jr., Ross, A.H., Connors, J.M., Fujiki, H., Sugimura, T., Rosner, M.R.: Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc. Natl. Acad. Sci. U. S. A. 81, 3034–3038 (1984)
Foulkes, J.G., Chow, M., Gorka, C., Frackelton, A.R. Jr., Baltimore, D.: Purification and characterization of a protein-tyrosine kinase encoded by the Abelson murine leukemia virus. J. Biol. Chem. 260, 8070–8077 (1985)
Daniel, T.O., Tremble, P.M., Frackelton, A.R. Jr., Williams, L.T.: Purification of the platelet-derived growth factor receptor by using an anti-phosphotyrosine antibody. Proc. Natl. Acad. Sci. U. S. A. 82, 2684–2687 (1985)
Bacon, K.B.: Chemokine Induced Signal Transduction in Leukocyte. Chemokine and Their Receptor: From Basic Research to Therapeutic Intervention. Institute Pasteur, Paris, France (1999)
Hommes, D.W., Peppelenbosch, M.P., van Deventer, S.J.: Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 52, 144–151 (2003)
Acknowledgement
Varun Kesherwani is CSIR-SRF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sodhi, A., Kesherwani, V. Production of TNF-α, IL-1β, IL-12 and IFN-γ in murine peritoneal macrophages on treatment with wheat germ agglutinin in vitro: involvement of tyrosine kinase pathways. Glycoconj J 24, 573–582 (2007). https://doi.org/10.1007/s10719-007-9054-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-007-9054-z