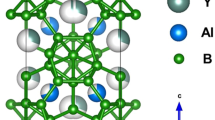
The processes involved in obtaining lanthanum hexaboride by boron thermal reduction using heat-treatment in a vacuum furnace and spark plasma sintering were investigated. Single-phase powder of lanthanum hexaboride was obtained at 1800 – 1900°C with excess boron content in the batch mix equal to 20 wt%. Spark plasma sintering allows the synthesis temperature of the single-phase lanthanum hexaboride powder to be lowered to 1700 and 1600°C with excess boron content 10 and 20 wt.%, respectively, in the batch.


















Similar content being viewed by others
References
E. N. Kablov, “Next-generation materials — the basis of innovation, technological leadership, and national security of Russia,” Intellekt Tekhnol., No. 2(14), 16 – 21 (2016).
E. N. Kablov, Trends and Landmarks for Russia’s Innovative Development: Information and Materials [in Russian], VIAM, Moscow (2015).
D. V. Grashchenkov, “Strategy for the development of non-metallic materials, metallic composite materials, and thermal protection,” Aviats. Mater. Tekhnol., No. S, 264 – 271 (2017). DOI: https://doi.org/10.8577/2071-9140-2017-0-S-264-271.
S. A. Evdokimov, N. E. Shchegoleva, and O. Yu. Sorokin, “Ceramic materials in aircraft engine building (review),” Trudy VIAM, Electron. Nauch.-Tekhn. Zh., No. 12, Art. 06 (2018). URL: http://www.viamworks.ru (date of access: 04/13/2022); DOI: https://doi.org/10.18577/2307-6046-2018-0-12-54-61.
D. O. Chukhvantsev, E. S. Filatov, N. I. Shurov, and D. A. Rozhentsev, “Synthesis of lanthanum hexaboride in chloride-oxide melt,” Neorg. Mater., 57(1), 16 – 21 (2021).
E. N. Kablov, D. A. Movenko, E. A. Lukina, et al., “Investigation of the structural-phase state of lanthanum hexaboride based ceramic material,” Glass Ceram., 76(11 – 12), 410 – 414 (2019).
C.-M. Chen, W.-C. Zhou, and L.-T. Zhang, “Oriented structure and crystallography of directionally solidified LaB6–ZrB2 eutectic,” J. Am. Ceram. Soc., 81(1), 237 – 240 (1998).
A. Taran, D. Voronovich, S. Plankovskii, et al., “Review of LaB6, Re-W dispenser, and BaHfO3-W cathode development,” TEEE Trans. Electron Devices, 56(5), P. 812 – 817.
J. T. Cahill and O. A. Graeve, “Hexaborides: a review of structure, synthesis and processing,” J. Mater. RES Technol., 8(6), 6321 – 6335 (2019).
T. Simsek, A. K. Chattopadhyay, M. Baris, and M. Bilen, “Low temperature synthesis and characterization of pure lanthanum hexaboride nanocrystals,” J. Solid State Chem., 276. 238 – 243 (2019).
J. K. Sonber, K. Sairam, T. S. R. Ch. Murthy, et al., “Synthesis, densification and oxidation study of lanthanum hexaboride,” J. Eurp. Ceram. Soc., 34(5), 1155 – 1160 (2014).
M. Jha, R. Patra, S. Ghosh, and A. K. Ganguli, “Novel borothermal route for the synthesis of lanthanum cerium hexaborides and their field emission properties,” J. Solid State Chem., 194, 173 – 178 (2012).
E. Sani, L. Mercatelli, M. Meucci, et al., “Lanthanum hexaboride for solar energy applications,” Sci. Rep., 7(1), Art. 718 (2017).
K. Uchida, “Cathodic Behavior in the Electrodeposition of LaB6,” Surf. Technol., 7(2), 137 – 143 (1978).
T. M. Mattox, Ch. Groome, A. Doran, et al., “Chloride influence on the formation of lanthanum hexaboride: An in-situ diffraction study,” J. Cryst. Growth, 486(1), 60 – 65 (2018).
M. S. Kuznetsov, Technology for Obtaining High-Emission Materials Based on Lanthanum Hexaboride in a Regime of Self-Propagating High-Temperature Synthesis with Mechanical Activation of the Charge, Author’s Abstract of Candidate’s Thesis [in Russian], National Research Tomsk Polytechnic University, Tomsk (2016).
Z. Dou, T. Zhang, Z. Zhang, et al., “Preparation and characterization of LaB6ultra fine powder by combustion synthesis,” Trans. Nonfer. Met. Soc. China, 21(8), 1790 – 1794 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 2, pp. 27 – 41, February, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kablov, E.N., Shchegoleva, N.E., Lebedeva, Y.E. et al. Lanthanum Hexaboride Production by Borothermal Reduction – an Investigation. Glass Ceram 80, 58–69 (2023). https://doi.org/10.1007/s10717-023-00557-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-023-00557-x