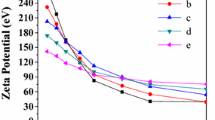
The physicochemical properties of the carboxylic cationite Tokem-200 and the sulfo-cationite Tokem-140 as well as their Ca2+-ion selectivity under equilibrium conditions were investigated and Tokem-200 based biomaterials of spherical form were synthesized from solutions by the sol-gel method. The material frame is represented by TiO2–SiO2; the inner part is filled with the oxide Ca2+ (sample TiO2–SiO2 /CaO). Unlike Tokem-140, the carboxylic cationite Tokem-200 possesses the highest Ca2+-ion selectivity, which makes it promising for the development of biomaterials. The solutions were found to remain suitable for up to 5 days for obtaining materials. To obtain a homogeneous material stepped heat-treatment is required (after drying at 60°C) at 150, 250, and 350°C (30 min at each step) and at 600°C for 6 h. Additional post-drying immersion of the samples into phosphoric acid does not favorably affect the properties of the material.






Similar content being viewed by others
References
A. Barba, A. Diez-Escudero, Y. Maazouz, et al., “Osteoinduction by foamed and 3D-printed calcium phosphate scaffolds: Effect of nanostructure and pore architecture,” ACS Appl. Mater. Interfaces, 9, 41722 – 41736 (2017).
X. Li, M. Wang, Y. Deng, et al., “Fabrication and properties of Ca–P bioceramic spherical granules with interconnected porous structure,” ACS Biomater. Sci. Eng., 3(8), 1557 – 1566 (2017).
S. H. Bjîrnîy, D. C. Bassett, S. Ucar, et al., “A correlative spatiotemporal microscale study of calcium phosphate formation and transformation within an alginate hydrogel matrix,” Acta Biomater., No. 44, 254 – 266 (2016).
M. Jokinen and H. Rahial, Relation between Aggregation and Heterogeneity of Obtained Structure in Sol-Gel Derived CaO–P 2O 5–SiO 2, Kluwer Academic Publishers, Dordrecht (1998), pp. 159 – 167.
H. Li and J. Chang, “Stimulation of proangiogenesis by calcium silicate bioactive ceramic,” Acta Biomater., No. 9, 5379 – 5389 (2013).
Y. Castro, J. Mosa, and M. Aparicio, “Sol-gel hybrid membranes loaded with meso/macroporous SiO2, TiO2–P2O5 and SiO2TiO2–P2O5 materials with high proton conductivity,” Mater. Chem. Phys., No. 149 – 150, 686 – 694 (2015).
Q. Wang, H. Hu, Y. Qiao, et al., “Enhanced performance of osteoblasts by silicon incorporated porous TiO2 coating,” J. Mater. Sci. Technol., No. 28, 109 – 117 (2012).
L. P. Borilo, T. S. Petrovskaya, and E. S. Lyutova, “Synthesis and properties of thin films based on phases of the SiO2–P2O5–CaO/system,” Neorg. Mater., 50(8), 874 – 877 (2014).
A. N. Shamsutdinova and V. V. Kozik, “Production and properties of thin films based on titanium, silicon and nickel oxides,”Khim. v Interesakh Ustoich. Razv., 24(5), 699 – 704 (2016).
E. A. Paukshtis, V. V. Kozik, A. S. Brichkovi, et al., “A method for producing a composite catalytic material in the form of layered hollow spheres, RF Pat. 2608125,” Byull. Izobr. Polezn. Modeli, No. 2, 10 (2017).
E. N. Gudymovich, M. A. Kiseleva, and L. N. Skvortsova, Complexometric Titration [in Russian], Tomsk (2000).
N. G. Polyansky, G. V. Gorbunov, and N. A. Polyanskaya, Methods for the Study of Ion Exchangers [in Russian], Khimiya, Moscow (1976).
A. S. Chugunov, “Acid-base properties of the Tokem-200 carboxylic cation exchanger during sorption of alkali metal and ammonium ions,” Izv. SPbGTI (TU), No. 29, 19 – 23 (2015).
V. V. Zharkova and L. A. Bobkova, “Concentration of manganese (II) and iron (III) ions from aqueous solutions by TOKEM and KB-2E cation exchangers,” Fundam. Issled., No. 8, Pt 2, 259 – 263 (2017).
R. K. Euler, Silica Chemistry [Russian translation], Mir, Moscow (1982).
L. P. Borilo, E. S. Lyutova, and L. N. Spivakova, “Study of biological properties of thin-film materials on the basis of the SiO2–P2O5–CaO SYSTEM,” Key Eng. Mater., 683, 427 – 432 (2016).
This work was performed as part of the state task No. 10.2281.2017_PCh.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 8, pp. 44 – 50, August, 2019.
Rights and permissions
About this article
Cite this article
Borilo, L.P., Kozik, V.V., Lyutova, E.S. et al. Sol-Gel Production and Properties of Spherical Biomaterials for the System TiO2–SiO2 /CaO. Glass Ceram 76, 315–320 (2019). https://doi.org/10.1007/s10717-019-00191-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-019-00191-6