Abstract
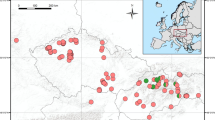
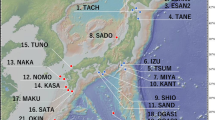
Using a cytological approach, diploid females were found coexisting with rare males in triploid apomictic parthenogenetic populations of the psyllid Cacopsylla myrtilli (W. Wagner, 1947) in Norway, Sweden, Finland and northwest Russia. Diploid females were easily distinguished from triploid apomictic females by the presence of 13 chiasmate bivalents instead of 39 univalent chromosomes at metaphase I. Abundance equaled that of males, but the proportion of males and diploid females was significantly greater in high altitude compared with low altitude populations. Males mated with females but showed no mating preference for diploid females. Lack of genuine bisexual reproduction owing to either asynaptic meiosis in males, or rarity of males with normal meiosis, suggests that diploids are produced in every generation by parthenogenetic females as reversals from triploidy, with their production being enhanced by environmental factor(s) associated with high altitude. This is further supported by the observation that within a population the COI haplotype found in rare males was the same as that in parthenogenetic triploid females. Thus, in northern Europe parthenogenesis in C. myrtilli is obligate, geographic parthenogenesis. Bisexual populations of C. myrtilli should be looked for in Central and Southern Europe. From the evolutionary point of view, the presence of males and diploid females with normal meiosis in parthenogenetic populations could be significant as they exhibit the potential to re-evolve either a new sexual species of parthenogenetic ancestry or a new parthenogenetic species by contagious parthenogenesis.

Similar content being viewed by others
References
Blackman RL (1972) The inheritance of life-cycle differences in Myzus persicae (Sulz.) (Hem: Aphididae). Bull Entomol Res 62:281–294
Blackman RL, Hales DF (1986) Behaviour of the X chromosomes during growth and maturation of parthenogenetic eggs of Amorpha tuberculate (Homoptera, Aphidae), in relation to sex determination. Chromosoma 94:59–64
Bloszyk J, Adamski Z, Napierala A, Dylewska M (2004) Parthenogenesis as a life strategy among mites of the suborder Uropodina (Acari: Mesostigmata). Can J Zool 82:1503–1511
Butlin R (2002) The costs and benefits of sex. New insights from old asexual lineages. Nat Rev Genet 3:311–317
Butlin RK, Shoen I, Martens K (1998) Asexual reproduction in nonmarine ostracods. Heredity 81:473–480
Crow JF (1994) Advantage of sexual reproduction. Dev Genet 15:205–213
D’Souza TG, Michiels NK (2010) The costs and benefits of occasional sex: theoretical predictions and a case study. J Hered 101(Suppl):34–41
Delmotte F, Leterme N, Bonhomme J, Rispe C, Simon J-C (2001) Multiple routes to asexuality in an aphid species. Proc Biol Sci 268:2291–2299
Domes K, Norton RA, Maraun M, Scheu S (2007) Re-evolution of sexuality breaks Dollo’s law. Proc Natl Acad Sci 104:7139–7144
Engelstädter J, Sandrock C, Vorburger C (2011) Contagious parthenogenesis, automixis, and a sex determination meltdown. Evolution 65:501–511
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Hodkinson ID (1976) New psyllids (Insecta: Homoptera: Psylloidea) from Canada. Zool J Linn Soc 58:321–330
Hodkinson ID (1978) The psyllids (Homoptera: Psylloidea) of Alaska. Syst Ent 3:333–360
Hodkinson ID (1983) Facultative parthenogenesis in Psylla myrtilli Wagner (Homoptera, Psyllidae): the saga continues in Norway. Fauna Norv Ser B 30:1–2
Hodkinson ID, Bird JM (2006) Facultative parthenogenesis in Cacopsylla myrtilli (Wagner) (Hemiptera: Psylloidea) in northern Sweden: possible explanations for the occurrence of males. Entomol Tidskr 127:157–160
Hurst LD, Peck JR (1996) Recent advances in understanding of the evolution and maintenance of sex. Trends Ecol Evol 11:46–52
Innes DJ, Herbert PDN (1988) The origin and genetic basis of obligate parthenogenesis in Daphnia pulex. Evolution 42:1024–1035
Kearney M (2005) Hybridization, glaciation and geographical parthenogenesis. Trends Ecol Evol 20:495–502
Klimaszewski SM (1971) Koliszki (Homoptera, Psylloidea) Bieszczadow. Fragm Faun 17:161–178
Kuznetsova VG, Labina ES, Shapoval NA, Maryanska-Nadachowska A, Lukhtanov VA (2012) Cacopsylla fraudatrix sp.n. (Hemiptera: Psylloidea) recognised from testis structure and mitochondrial gene COI. Zootaxa 3547:55–63
Labina ES, Nokkala S, Maryańska-Nadachowska A, Kuznetsova VG (2009) The distribution and population sex ratio of Cacopsylla myrtilli (W. Wagner, 1947) (Hemiptera: Psylloidea). Folia Biol (Kraków) 57:157–163
Lundmark M (2006) Polyploidization, hybridization and geographical parthenogenesis. Trends Ecol Evol 21:9
Lynch M (1984) Destabilizing hybridization, general purpose genotypes and geographic parthenogenesis. Quart Rev Biol 59:257–290
Maccari M, Gómez A, Hontoria F, Amat F (2013) Functional rare males in diploid parthneogenetic Artemia. J Evol Biol 26:1934–1948
Maccari M, Amat F, Hontoria F, Gómez A (2014) Laboratory generation of new parthenogenetic lineages supports contagious parthenogenesis in Artemia. PeerJ 2:439. doi:10.7717/peerj.439
Martens K (1998) Sex and ostracods: a new synthesis. In: Schön I, Martens K (eds) Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine ostracods. Backhuys Publishers, Leiden, pp 295–322
Neiman M, Larkin K, Thompson AR, Wilton P (2012) Male offspring production by asexual Potamopyrgus antipodarum, a New Zealand snail. Heredity 109:57–62
Nokkala S, Maryańska-Nadachowska A, Kuznetsova VG (2008) First evidence of polyploidy in Psylloidea (Homoptera, Sternorrhyncha): a parthenogenetic population of Cacopsylla myrtilli (W. Wagner, 1947) from northeast Finland is apomictic and triploid. Genetica 133:201–205
Nokkala C, Kuznetsova V, Nokkala S (2013) Meiosis in rare males in parthenogenetic Cacopsylla myrtilli (Wagner, 1947) (Hemiptera, Psyllidae) population from northern Europe. Comp Cytogenet 7:241–251
Normark BB (2003) The evolution of alternative genetic systems in insects. Annu Rev Entomol 48:397–423
Normark BB, Judson OP, Moran NA (2003) Genomic signatures of ancient asexual lineages. Biol J Linn Soc 79:69–84
Paland S, Colbourne JK, Lynch M (2005) Evolutionary history of contagious asexuality in Daphnia pulex. Evolution 59:800–813
Palmer SC, Norton RA (1990) Further experimental proof of thelytokous parthenogenesis in Oribatid mites (Acari: Oribatida: Desmonomata). Exp Appl Acarol 8:149–159
Rispe C, Bonhomme J, Simon J-C (1999) Extreme lifecycle and sex ratio variation among sexually produced clones of the aphid Rhopalosiphum padi (Homoptera: Aphididae). Oikos 86:254–264
Schön I, Martens K, Van Dijk P (eds) (2009) Lost sex: the evolutionary biology of parthenogenesis. Springer, Dordrecht
Simon J-C, Leterme N, Latorre A (1999) Molecular markers linked to breeding system differences in segregating and natural populations of the cereal aphid Rhopalosiphum padi L. Mol Ecol 8:965–973
Simon J-C, Delmotte F, Rispe C, Crase T (2003) Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol J Linn Soc 79:151–163
Snyder DW, Opperman CH, Bird DM (2006) A method for generating Meloidogyne incognita males. J Nematol 38:192–194
Suomalainen E, Saura A, Lokki J (1987) Cytology and evolution in parthenogenesis. CRC Press, Boca Raton
Taberly G (1988) Recherches sur la parthénogenèse thélytoque de deux espèces d’acariens oribatides: Trypochthonius tectorum (Berlese) et Platynothrus peltifer (Koch). IV. Observation sur les males ataviques. Acarologia 29:95–107
Wahlberg N, Wheat CW (2008) Genomic outposts serve the phylogenomic pioneers: designing novel nuclear markers for genomic DNA extractions of Lepidoptera. Syst Biol 57:231–242
Acknowledgments
We wish to express our thanks to Prof N. Wahlberg and two anonymous reviewers for valuable comments on the manuscript and T. Helenius and E. Labina for technical assistance in making cytological preparations. This work was supported by the University of Turku Foundation project grant to SN and CN and by the Russian Foundation for Basic Research (Grant 14-04-01051) to VK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nokkala, C., Kuznetsova, V.G. & Nokkala, S. Rare diploid females coexist with rare males: a novel finding in triploid parthenogenetic populations in the psyllid Cacopsylla myrtilli (W. Wagner, 1947) (Hemiptera, Psylloidea) in northern Europe. Genetica 143, 589–595 (2015). https://doi.org/10.1007/s10709-015-9858-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-015-9858-x