Abstract
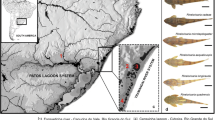
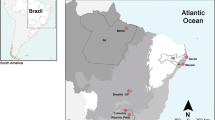
Structural chromosome changes are widely described in different vertebrate groups and generate genetic, phenotypic and behavioral diversity. During the evolution of loricariids, several rearrangements (fissions, fusions, inversions) seem to have occurred. Hypancistrus, tribe Ancistrini, are highly demanded for fishkeeping around the world. In this tribe, the diploid chromosome number 2n = 52 is considered a synapomorphy, and paracentric-type inversions appear to be involved in the chromosomal evolution of the tribe. The present study investigated the karyotypes of H. zebra and H. cf. debilittera using cytogenetic, classical and molecular tools, as well as DNA barcoding. Data reveal that, although diploid number in both species corroborates the proposed synapomorphy for the tribe, there is a complex karyotype dynamics, reflected in the intense chromosomal polymorphism, resulting from rearrangements involving ribosomal regions (5S and 18S rDNA), which are suggested to be paracentric inversions. Besides, DNA barcode confirms reciprocal monophyletism between the species, validating the existence of two species, only. This scenario, coupled with genomic instability caused by exogenous sequences such as Rex-3 retrotransposons and the species’ sedentary lifestyle, which helps the fast polymorphism fixation, may reflect different phenotypic color patterns in natural populations, as observed in H. cf. debilittera.








Similar content being viewed by others
References
Alves AL, Oliveira C, Foresti F (2003) Karyotype variability in eight species of the subfamilies Loricariinae e Ancistrinae (Teleostei, Siluriformes, Loricariidae). Caryologia 56:57–63
Armbruster JW (2004) Phylogenetic relationships of the suckermouth armoured catfishes Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. Zool J Linn Soc 141:1–80
Armbruster J, Lujan NK, Taphorn DC (2007) Four new Hypancistrus (Siluriformes: Loricariidae) from Amazonas, Venezuela. Copeia 1:62–79
Artoni RF, Bertollo LAC (2001) Trends in the karyotype evolution of Loricariidae fish (Siluriformes). Hereditas 134:201–210
Bellafronte E, Schemberger MO, Artoni RF, Moreira-Filho O, Vicari MR (2012) Sex chromosome system ZZ/ZW in Apareiodon hasemani Eigenmann, 1916 (Characiformes, Parodontidae) and a derived chromosomal region. Genet Mol Biol 35:770–776. doi:10.1590/S1415-47572012005000077
Bertollo LAC, Takahashi CS, Moreira-Filho O (1978) Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Rev Bras Genet 1:103–120
Bueno V, Zawadzki CH, Margarido VP (2012) Trends in chromosome evolution in the genus Hypostomus Lacepede, 1803 (Osteichthyes, Loricariidae): a new perspective about the correlation between diploid number and chromosomes types. Rev Fish Biol Fish 22:241–250. doi:10.1007/s11160-011-9215-9
Capistano TG, Castro ALBP, Júlio-Junior HF (2008) Chromosome divergence and NOR polymorphism in Bryconamericus aff. iheringii (Teleostei, Characidae) in the hydrographic systems of Paranapanema and Ivaí Rivers, Paraná, Brazil. Genet Mol Biol 31:203–207
Cardoso AL, Sales KAH, Nagamachi CY, Pieczarka JC, Noronha RCR (2013) Comparative cytogenetics of two species of genus Scobinancistrus (Siluriformes, Loricariidae, Ancistrini) from the Xingu River, Brazil. Comp Cytogen 7:43–51. doi:10.3897/CompCytogen.v7i1.4128
Collins RA, Armstrong KF, Meier R, Yi Y, Brown SDJ, Cruickshank RH, Keeling S, Johnston C (2012) Barcoding and border biosecurity: identifying cyprinid fishes in the aquarium trade. PLoS ONE 7(1):e28381. doi:10.1371/journal.pone.0028381
de Oliveira RR, Feldberg E, Anjos MB, Zuanon J (2007) Karyotype characterization and ZZ/ZW sex chromosomes heteromorphism in two species of the catfish genus Ancistrus Kner, 1854 (Siluriformes: Loricariidae) from the Amazon basin. Neotrop Ichthyol 5:301–306. doi:10.1590/S1679-62252007000300010
de Oliveira RR, Feldberg E, Anjos MB, Zuanon J (2008) Mechanisms of chromosomal evolution and its possible relation to natural history characteristics in Ancistrus catfishes (Siluriformes: Loricariidae). J Fish Biol 75:2209–2225. doi:10.1111/j.1095-8649.2009.02450.x
Elgin SCR (1996) Heterocromatin and gene regulation in Drosophila. Genetics Dev 6:193–202
Fenocchio AS, Bertollo LAC (1992) Karyotype similarities among Pimelodidae (Pisces, Siluriformes) from the Brazilian Amazon region. Cytobios 69:41–46
Ferreira DC, Oliveira C, Foresti F (2011) A new dispersed element in the genome of Hisonotus leucofrenatus (Teleostei: Siluriformes: Hypoptopomatinae). Mob Genet Elements 1:1–4. doi:10.4161/mge.1.2.17450
Fish-Muller S, Montoya-BurgosJI, Le Bail PY, Covain R (2012) Diversity of the Ancistrini (Siluriformes: Loricariidae) from the Guianas: the Panaque group, a molecular appraisal with descriptions of new species. In: Covain R, Fisch-Muller S (eds), Fishes of the Guianas: scientific advances and future prospects for a highly diversified fauna. Cybium, vol 36, issue no. 1, pp 163–193
Garcia C, Moreira-Filho O (2008) Localization of ribosomal genes in three Pimelodus species (Siluriformes, Pimelodidae) of the São Francisco River: 5S genes as species markers and conservation of the 18S rDNA sites. Genet Mol Biol 31:261–264. doi:10.1590/S1415-47572008000200018
Giuliano-Caetano L (1998) Polimorfismo cromossômico Robertsoniano em populações de Rineloricaria latirostris (Pisces, Loricariidae). PhD Thesis. Universidade Federal de São Carlos, p 78
Gross MC, Feldberg E, Cella DM, Schneider MC, Schneider CH, Porto JIR, Martins C (2009) Intriguing evidence of translocations in Discus fish (Symphysodon, Cichlidae) and a report of the largest meiotic chromosomal chain observed in vertebrates. Heredity 102:435–441. doi:10.1038/hdy.2009.3
Gross MC, Schneider CH, Valente GT, Martins C, Feldberg E (2010) Variability of 18S rDNA locus among Symphysodon fishes: chromosomal rearrangements. J Fish Biol 76:1117–1127. doi:10.1111/j.1095-8649.2010.02550.x
Grutzner F, Rens W, Tsend-Ayush E, El-Mogharbel N, O’Brien PC, Jones RC et al (2004) In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432:913–917. doi:10.1038/nature03021
Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B 270:313–321. doi:10.1098/rspb.2002.2218
Howell WM, Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a I-step method. Experientia 36:1014–1015
Inácio A, Pinho J, Comai L, Coelho MM (2012) Global analysis of the small RNA transcriptome in different ploidies and genomic combinations of a vertebrate complex—the Squalius alburnoides. PLoS ONE 7:1–10. doi:10.1371/journal.pone.0041158
Isbrucker IJH, Nijssen H (1991) Hypancistrus zebra, a new genus and species of uniquely pigmented Ancistrine Loricariidae from the Rio Xingu, Brazil (Pisces: Siluriformes: Loricariidae). Ichthyoll Explor Fres 1:345–350
Jesus CM, Moreira-Filho O (2000) Cytogenetic studies in some Apareiodon species (Pisces, Parodontidae). Cytologia 65:397–402
Kavalco KF, Pazza R, Bertollo LCB, Moreira-Filho O (2004) Heterochromatin characterization of four fish species of the family Loricariidae (Siluriformes). Hereditas 141:237–242. doi:10.1111/j.1601-5223.2004.01850.x
Kavalco KF, Pazza R, Bertollo LAC, Moreira-Filho O (2005) Karyotypic diversity and evolution of Loricariidae (Pisces, Siluriformes). Heredity 94:180–186. doi:10.1038/sj.hdy.6800595
Kerr KCR, Stoeckle MY, Dove CJ, Weigt LA, Francis CM, Hebert PDN (2007) Comprehensive DNA barcode coverage of North American birds. Mol Ecol Notes 7:535–543. doi:10.1111/j.1471-8286.2007.01670.x
Kidwell MG (2002) Transposable elements and the evolution of genome size in eukaryotes. Genetica 115:49–63
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kobayashi T (2008) A new role of the rDNA and nucleolus in the nucleus—rDNA instability maintains genome integrity. Bio Essays 30:267–272. doi:10.1002/bies.20723
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Machado TC, Pansonato-Alves JC, Pucci MB, Nogaroto V, Almeida MC, Oliveira C et al (2011) Chromosomal painting and ZW sex chromosomes differentiation in Characidium (Characiformes, Crenuchidae). BMC Genet 12:65. doi:10.1186/1471-2156-12-65
Mariotto S, Centofante L, Miyazawa CS, Bertollo LAC, Moreira-Filho O (2009) Chromosome polymorphism in Ancistrus cuiabae Knaack, 1999 (Siluriformes, Loricariidae, Ancistrini). Neotrop Ichthyol 7:595–600. doi:10.1590/S1679-62252009000400006
Mariotto S, Centofante L, Vicari MR, Artoni RF, Moreira-Filho O (2011) Chromosomal diversification in ribosomal DNA sites in Ancistrus Kner, 1854 (Loricariidae, Ancistrini) from three hydrographic basins of Mato Grosso, Brazil. Comp Cytogen 5:289–300. doi:10.3897/CompCytogen.v5i4.1757
Mark K, Barton N (2005) Chromosome inversions, local adaptation and speciation. Genetics 173:419–434. doi:10.1534/genetics.105.047985
Martins C, Galetti PM Jr (1999) Chromosomal localization of 5S rDNA genes in Leporinus fish (Anostomidae, Characiformes). Chromosome Res 7:363–367
Matoso DA, Almeida-Val VMF, Silva M, Moraes-Neto A, Almeida MC, Vicari MR et al (2011) Chromosomal polymorphism in Steindachneridion melanodermatum Garavello, 2005 (Siluriformes, Pimelodiade): a reappraisal the existence of sex chromosome system in the species. Rev Fish Biol Fish 21:497–508. doi:10.1007/s11160-011-9201-2
Mazzuchelli J, Martins C (2009) Genomic organization of repetitive DNAs in the cichlid fish Astronotus ocellatus. Genetica 136:461–469. doi:10.1007/s10709-008-9346-7
Mendes Neto EOM, Vicari MR, Artoni RF, Moreira-Filho O (2011) Description of karyotype in Hypostomus regani (Ihering, 1905) (Teleostei, Loricariidae) from the Piumhi river in Brazil with comments on karyotype variation found in Hypostomus. Comp Cytogen 5:133–142. doi:10.3897/compcytogen.v5i2.964
Montoya-Burgos JI (2003) Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Mol Ecol 12(7):1855–1867. doi:10.1046/j.1365-294X.2003.01857.x
Pansonato-Alves JC, Vicari MR, Oliveira C, Foresti F (2011) Chromosomal diversification in populations of Characidium cf. gomesi (Teleostei, Crenuchidae). J Fish Biol 78:183–194. doi:10.1111/j.1095-8649.2010.02847.x
Pereira LH, Hanner R, Foresti F, Oliveira C (2013) Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC genetics 14(1):20. doi:10.1186/1471-2156-14-20
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83:2934–2938
Reis DAR, Brandão KO, Almeida-Toledo LF, Pazza R, Kavalco KF (2012) Physical location of ribosomal genes 5S and 18S in Ancistrus sp. (Loricariidae: Ancistrini) from Angra dos Reis—RJ, coastal river basin. Evol Conserv Biodivers 3:39–44
Ribeiro LB, Matoso DA, Almeida MC, Vicari MR, Moraes-Neto A, Svidnicki MCCM, Artoni RF (2008) Karyotypic variability in Iheringichthys labrosus (Teleostei, Pimelodidae) from the Tibagi River basin (Paraná State, Brazil). Genet Mol Res 7:718–724
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Nati Acad Sci USA 74(12):5463–5467
Schemberger MO, Bellafronte E, Nogaroto V, Almeida MC, Schuhli GS, Artoni RF et al (2011) Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica 139:1499–1508. doi:10.1007/s10709-012-9649-6
Scouarnec SL, Gribble SM (2012) Characterising chromosome rearrangements: recent technical advances in molecular cytogenetics. Heredity 108:75–85. doi:10.1038/hdy.2011.100
Silva M, Matoso DA, Vicari MR, de Almeida MC, Margarido VP, Artoni RF (2011) Physical mapping of 5S rDNA in two species of Knifefishes: Gymnotus pantanal and Gymnotus paraguensis (Gymnotiformes). Cytogenet Genome Res 134:303–307. doi:10.1159/000328998
Souza CAP, Nascimento LA, Carvalho-Jr RJ, Barros SMR, Feldberg E, Nagamachi CY, Pieczarka JC (2004) Karyotypic analysis of Baryancistrus aff. niveatus (Ancistrinae, Loricariidae) by C-banding, Ag-NOR, CMA3, DAPI and FISH. Caryologia 57(3):219–223
Souza EMS, Silva CEF, Eler ES, Silva MNF, Feldberg E (2013) Variations of chromosomal structures in Caluromys philander (Didelphimorphia: Didelphidae) from Amazon region. Genetica Online. doi:10.1007/s10709-013-9708-7
Sumner AT (1972) A Simple technique for demonstrating centromeric heterocromatin. Exp Cell Res 75:304–306
Sumner AT (1990) Chromosome banding. Unwin Hyman, Londres
Swarça AC, Cestari MM, Giuliano-Caetano L, Dias AL (2001) Cytogenetic characterization of the large South American siluriform fish species Zungaro zungaro (Pisces, Pimelodidae). Chromosome Sci 5:51–55
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Terencio ML, Schneider CH, Gross MC, Vicari MR, Feldberg E (2012) Stable karyotypes: a general rule for the fish of the family Prochilodontidae? Hydrobiologia 686:147–156. doi:10.1007/s10750-012-1006-3
Thomas JW, Cácares M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL et al (2008) The chromosomal polymorphism linked to variation in social behavior in the white-troated sparrow (Zonotrichia albicolis) is a complex rearrangement and suppressor of recombination. Genetic Soc Am 179:1455–1468. doi:10.1534/genetics.108.088229
Valente GT, Mazzuchelli J, Ferreira IA, Poletto AB, Fantinatti BEA, Martins C (2011) Cytogenetic mapping of the retroelements Rex1, Rex3 and Rex6 among cichlid fish: new insights on the chromosomal distribution of transposable elements. Cytogenet Genome Res 133:34–42. doi:10.1159/000322888
Vences M, Thomas M, van der Meijden A, Chiari Y, Vieites DR (2005) Comparative performance of the 16S rRNA gene in DNA barcoding of amphibians. Front Zool 2(1):5. doi:10.1186/1742-9994-2-5
Vicari MR, Moreira-Filho O, Artoni RF, Bertollo LAC (2006) ZZ/ZW sex chromosome system in an undescribed species of the genus Apareiodon (Characiformes, Parodontidae). Cytogenet Genome Res 114:163–168. doi:10.1159/000093333
Vicari MR, Nogaroto V, Noleto RB, Cestari MM, Cioffi MB, Almeida MC et al (2010) Satellite DNA and chromosomes in Neotropical fishes: methods, applications and perspectives. J Fish Biol 76:1094–1116. doi:10.1111/j.1095-8649.2010.02564.x
Volff JN, Korting C, Sweeney K, Schartl M (1999) The non-LTR Rex3 from the fish Xiphophorus is widespread among teleosts. Mol Biol Evol 16:1427–1438
Ward RD, Zemlak SZ, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Phil Trans R Soc B 360:1847–1857. doi:10.1098/rstb.2005.1716
Whakimoto BT, Hearn MG (1990) The effects of chromosome rearrangements on the expression of heterochromatic genes in chromosome 2L of Drosophila melanogaster. Genetics 125:141–154
Zhimulev IF, Belyaeva ES, Formina OV, Protopopov MO, Bolshakov VN (1986) Cytogenetic and molecular aspects of position effect variegation in Drosophila melanogaster. I. Morphology and genetic activity of the 2AB region in chromosome rearrangement T (1;2) dorvar7. Chromosoma 94:492–504
Ziemniczak K, Barros AC, Rosa KO, Nogaroto V, Almeida MC, Cestari MM et al (2012) Comparative cytogenetics of Loricariidae (Actinopterygii: Siluriformes): emphasis in Neoplecostominae and Hypoptopomatinae. Ital J Zool 79:1–10. doi:10.1080/11250003.2012.676677
Acknowledgments
The authors are grateful to IBAMA for the donation of the specimens. This study was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq) and Research Support Foundation of the State of Amazonas (Fundação de Amparo à Pesquisa do Estado do Amazonas). Centre for Studies of Adaptation to Environmental Changes in the Amazon (INCT ADAPTA,FAPEAM/CNPq 573976/2008-2), PRONEX/FAPEAM/CNPQ 003/2009). LMS was funded by CNPq-510 PROTAX (150044/2011-9). The authors would also like to thank the biologist Leandro Marajó da Silva for help with the analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, M., Ribeiro, E.D., Matoso, D.A. et al. Chromosomal polymorphism in two species of Hypancistrus (Siluriformes: Loricariidae): an integrative approach for understanding their biodiversity. Genetica 142, 127–139 (2014). https://doi.org/10.1007/s10709-014-9760-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-014-9760-y