Abstract
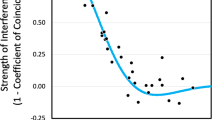
The mus309 gene in Drosophila melanogaster encodes a RecQ helicase which is involved in DNA double-strand break (DSB) repair. In a brood pattern analysis, it was observed that in mus309 mutant females, the frequency of single crossovers in the central cv–v interval of the X chromosome was reduced in young females but returned to the level of the wild type control as the females aged. In the proximal v–f interval, the frequency of single crossovers was increased during the entire experimental period. In particular, it was observed that the frequency of double crossovers, as well as the coefficient of coincidence first increased but then gradually decreased, finally reaching the level of the control flies, as the females aged. Map distances increased due to the mus309 mutation in both gene interval studies, but they did not change as the females aged, a result suggesting that the mus309 gene controls the distribution of DSBs to be repaired as crossovers instead of non-crossovers. The results suggest a mechanism for the centromere effect of crossing over in Drosophila, viz the fact the frequency of meiotic crossing over reduces with the age of the female, and that the reduction is more pronounced the closer the interval is to the proximal heterochromatin of the chromosome arm. According to the model suggested, the centromere effect is simply a matter of the balance between different pathways of the repair of the DSBs of DNA.







Similar content being viewed by others
References
Adams MD, McVey M, Sekelsky JJ (2003) Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299:265–267
Baker BS, Carpenter ATC (1972) Genetic analysis of sex chromosomal meiotic mutants in Drosophila melanogaster. Genetics 71:255–286
Baker BS, Hall JC (1976) Meiotic mutants: Genetic control of meiotic recombination and chromosome segregation. In: Ashburner M, Novitski E (eds) The genetics and biology of Drosophila, vol 1a. Academic Press, London, pp 351–434
Beadle GW (1932) A possible influence of spindle fibre on crossing-over in Drosophila. Proc Natl Acad Sci USA 18:160–165
Beal EL, Rio DC (1996) Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 gene and is involved in P-element excision in vivo. Genes Dev 10:921–933
Boyd JB, Golino MD, Shaw KES, Osgood CJ, Green MM (1981) Third-chromosome mutagen-sensitive mutants of Drosophila melanogaster. Genetics 97:607–623
Boyd JB, Mason JM, Yamamoto AH, Brodberg RK, Banga SS et al (1987) A genetic and molecular analysis of DNA-repair in Drosophila. J Cell Sci Suppl 6:39–60
Bridges CB (1915) A linkage variation in Drosophila. J Exp Zool 16:1–15
Bridges CB (1927) The relation of the age of the female to crossing over in the third chromosome of Drosophila melanogaster. J Gen Physiol 8:687–700
Ellis NA, Groden J, Ye T-Z, Straughen J, Lennon DJ et al (1995) The Bloom’s syndrome gene-product is homologous to RecQ helicases. Cell 83:655–666
Grell RF (1973) Recombination and DNA replication in the Drosophila melanogaster oocyte. Genetics 73:87–108
Heyer W-D (2004) Recombination: Holliday junction resolution and crossover formation. Current Biol 14:R56–R58
Heyer W-D, Ehmsen KT, Solinger JA (2003) Holliday junctions in eukaryotic nucleus: resolution in sight? Trends Biochem Sci 28:548–557
Hillers KJ (2004) Crossover interference. Curr Biol 14:R1036–R1037
Johnson-Schlitz D, Engels WR (2006) Template disruption and failure of double Holliday junction dissolution during double-strand break repair in Drosophila BLM mutants. Proc Natl Acad Sci USA 103:16840–16845
Karow JK, Chakraverty RK, Hickson JD (1997) The Bloom’s syndrome gene product is a 3′–5′ DNA helicase. J Biol Chem 272:30611–30614
Keeney S (2001) Mechanism and control of meiotic recombination. Curr Top Dev Biol 52:1–53
Keeney S, Neale MJ (2006) Initiation of meiotic recombination by formation of DNA double-strand breaks: mechanism and regulation. Biochem Soc Trans 34:523–525
Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88:375–384
Kusano K, Johnson-Schlitz DM, Engels WR (2001) Sterility of Drosophila with mutations in the Bloom syndrome gene—complementation by Ku70. Science 291:2600–2602
Lake S (1984) Variation in the recombination frequency and the relationship to maternal age in brood analysis of the distal and centromeric regions of the X-chromosome in temperature shocked reciprocal hybrids of inbred lines of Drosophila melanogaster. Hereditas 100:121–129
Laurencon A, Orme CM, Peters HK, Boulton CL, Vladar EK et al (2004) A large-scale screen for mutagen sensitive loci in Drosophila. Genetics 167:217–231
Lorenz A, Whitby MC (2006) Crossover promotion and prevention. Biochem Soc Trans 34:537–541
Lüning KG (1983) Genetics of inbred Drosophila melanogaster X. Maternal and cytoplasmic effects on recombination. Hereditas 99:57–68
Mather K (1939) Crossing over and heterochromatin in the X chromosome of Drosophila melanogaster. Genetics 24:413–435
McVey M, Andersen SL, Broze Y, Sekelsky JJ (2007) Multiple functions of Drosophila Blm helicase in maintenance of genome stability. Genetics 176:1979–1992
Mohaghegh P, Karow JK, Brosh RM Jr, Bohr VA, Hickson ID (2001) The Bloom’s and Werner’s syndrome proteins are DNA structure-specific homologues. Nucl Acids Res 29:2843–2849
Muller HJ (1916) The mechanism of crossing over. Am Nat 50:193–221
Olsen-Krogh B, Symington LS (2004) Recombination proteins in yeast. Ann Rev Genet 38:233–271
Portin P (2005) mus309 mutation, defective in DNA double-strand break repair, affects intergenic but not intragenic meiotic recombination in Drosophila melanogaster. Genet Res 86:185–191
Portin P (2008) Corrigendum: mus309 mutation, defective in DNA double-strand break repair, affects intergenic but not intragenic meiotic recombination in Drosophila melanogaster. Genet Res 90:297
Rockmill B, Fung JC, Branda SS, Roeder GS (2003) The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr Biol 13:1954–1962
Sandler L, Lindsley DL, Nicoletti B, Trippa G (1968) Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics 60:525–558
Stevens WL (1936) The analysis of interference. J Genet 32:51–64
Sturtevant AH (1915) The behavior of the chromosomes as studied through linkage. Z Induc Abstam Vererb 13:234–287
van Brabant AJ, Stan R, Ellis NA (2000) DNA helicases, genome instability, and human genetic disease. Ann Rev Genom Hum Genet 1:409–459
Weinert BT, Rio DC (2007) DNA strand displacement, strand annealing and strand swapping by the Drosophila Bloom’s syndrome helicase. Nucl Acids Res 35:1367–1376
Weinstein A (1936) The theory of multiple-strand crossing over. Genetics 21:155–199
Wu L, Davies SL, Levitt NC, Hickson ID (2001) Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51. J Biol Chem 276:19375–19381
Acknowledgments
Thanks are given to Professor Janos Szabad (Szeged, Hungary) for introducing me to the mus309 gene, and the generous donation of the mutant stocks. Skilful technical assistance by Mirja Rantanen, M.Sc. is gratefully acknowledged. Docent Kai Ruohomäki, PhD, and Mia Rönkä, M.Sc., helped me by conducting the statistical analyses, and Kurt Ståhle, the technician, by drawing the figures. To all of them I am very grateful. Special thanks are given to Maaria Tringham, M.Sc., and Damon Tringham, M.Phil., for checking the language.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was retracted due to plagiarism.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10709-010-9519-z
About this article
Cite this article
Portin, P. RETRACTED ARTICLE: The effect of the mus309 mutation, defective in DNA double-strand break repair, on crossing over in Drosophila melanogaster suggests a mechanism for the centromere effect of crossing over. Genetica 138, 333–342 (2010). https://doi.org/10.1007/s10709-009-9422-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-009-9422-7