Abstract
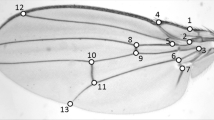
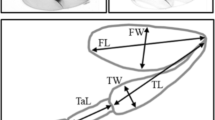
To investigate the size and shape of the aedeagus of Drosophila mediopunctata, we used basic statistics and geometric morphometrics. We estimated the level of phenotypic variation, natural and laboratory heritability as well as the phenotypic correlations between aedeagus and wing measures. The wing was used as an indicator for both body size and shape. Positive significant correlation was obtained for centroid size of aedeagus and wing for field parents and their offspring reared in the laboratory. Many positive significant phenotypic correlations were found among linear measures of both organs. The phenotypic correlations were few for aedeagus and wing shape. Coefficients of variation of the measures were on average larger in the aedeagus than in the wing for offspring reared in laboratory, but not for flies coming from the field. Significant “natural” heritabilities were found for five linear measures of the aedeagus and only one for the wing. Few significant heritabilities were found for aedeagus and wing shape, mostly ones concerning the uniform components. In an exploratory analysis, we found that inversion DS-PC0 is associated with both uniform and nonuniform components of shape, respectively, in the wing and aedeagus. Our results do not support the lock-and-key hypothesis for the male genitalia evolution, but cannot refute the sexual selection and pleiotropy hypotheses.



Similar content being viewed by others
Abbreviations
- \( {\text{h}}^{2}_{{\text{n}}} \) :
-
Natural heritability
- \( {\text{h}}^{2}_{{{\text{lab}}}} \) :
-
Laboratory heritability
- AHa, CGa, CIa, DHa, etc.:
-
Linear measures of the aedeagus
- ABw, BCw, CDw, etc.:
-
Linear measures of the wing
- RW:
-
Relative warp
- Cs:
-
Centroid size
References
Ananina G, Peixoto AA, Souza WN et al (2002) Polytene chromosome map and inversion polymorphism in Drosophila mediopunctata. Mem Inst Oswaldo Cruz 97:691–694
Ananina G, Peixoto AA, Bitner-Mathé BC et al (2004) Chromosomal inversion polymorphism in Drosophila mediopunctata: seasonal, altitudinal, and latitudinal variation. Genet Mol Biol 27:61–69
Andrade CAC, Hatadani LM, Klaczko LB (2005) Phenotypic plasticity of the aedeagus of Drosophila mediopunctata. J Therm Biol 30:518–523
Arnqvist G (1997) The evolution of animal genitalia: distinguishing between hypotheses by single species studies. Biol J Linn Soc 60:365–379
Arnqvist G (1998) Comparative evidence for the evolution of genitalia by sexual selection. Nature 393:784–786
Arnqvist G, Thornhill R (1998) Evolution of animal genitalia: patterns of phenotypic and genotypic variation and condition dependence of genital and non-genital morphology in water strider. Genet Res Camb 71:193–212
Bertin A, Fairbairn DJ (2007) The form of sexual selection on male genitalia cannot be inferred from within-population variance and allometry – a case study in Aquarius remigis
Bitner-Mathé BC, Klaczko LB (1999a) Size and shape heritability in natural populations of Drosophila mediopunctata: temporal, microgeographical variation. Genetica 105:35–42
Bitner-Mathé BC, Klaczko LB (1999b) Heritability, phenotypic and genetic correlations of size and shape of Drosophila mediopunctata wings. Heredity 83:688–696
Bitner-Mathé BC, Klaczko LB (1999c) Plasticity of Drosophila melanogaster wing morphology: effects of sex, temperature and density. Genetica 105:203–210
Bitner-Mathé BC, Peixoto AA, Klaczko LB (1995) Morphological variation in a natural population of Drosophila mediopunctata: altitudinal cline, temporal changes and influence of chromosome inversions. Heredity 75:54–61
Bond JE, Beamer DA, Hedin MC et al (2003) Gradual evolution of male genitalia in a sibling species complex of millipedes (Diplopoda: Spirobolida: Rhinocricidae: Anadenobolus). Invert Syst 17:711–717
Bookstein FL (1991) Morphometric tools for landmark data. Geometry and biology. Cambridge University Press, New York
Breuer ME, Pavan C (1950) Genitália masculina de Drosophila (Diptera): grupo annulimana. Rev Bras Biol 10:469–488
Breuer ME, Pavan C (1954) Genitália masculina de Drosophila do grupo dreyfusi (Diptera). Rev Bras Biol 14:465–475
Coyne JA, Beecham E (1987) Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics 117:727–737
DeCarvalho AB, Peixoto AA, Klaczko LB (1989) Sex-ratio in Drosophila mediopunctata. Heredity 62:425–428
Dobzhansky Th, Pavan C (1943) Studies on Brazilian species of Drosophila. Bol. Fac. Fil. Cien. Let., Univ S. Paulo, XXXVI. Biologia Geral 36:7–72
Eberhard WG (1985) Sexual selection and animal genitalia. Harvard University Press, Cambridge, Mass
Eberhard WG (1990) Animal genitalia and female choice. Amer Sci 78:134–141
Eberhard WG (1993) Evaluating models of sexual selection: genitalia as a test case. Am Nat 142:564–571
Eberhard WG (2001) Species-specific genitalic copulatory courtship in sepsid flies (Diptera, Sepsidae, Microsepsis). Evolution 55:93–102
Eberhard WG (2004) Rapid divergent evolution of sexual morphology: comparative tests of antagonistic coevolution and traditional female choice. Evolution 58:1947–1970
Eberhard WG (2005) Sexual morphology of male Sepsis cynipsea (Diptera: Sepsidae): lack of support for lock-and-key and sexually antagonistic morphological coevolution hypotheses. Can Entomol 137:551–565
Falconer DS (1981) Introduction to quantitative genetics. 2nd edn. Longman, Essex
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics. Longman, Essex
Franco FF, Prado PRR, Sene FM et al (2006) Aedeagus morphology as a discriminant marker in two closely related Cactophilic species of Drosophila (Diptera; Drosophilidae) in South America. An Acad Bras Ciênc 78:203–212
Gilchrist AA, Partridge L (2001) The contrasting genetic architecture of wing size and shape in Drosophila melanogaster. Heredity 86:144–152
Haas HL, Tolley KA (1998) Geographic variation of wing morphology in three Eurasian populations of the fruit fly, Drosophila lummei. J Zool (London) 245:197–203
Hatadani LM, Baptista JCR, Souza WN et al (2004) Colour polymorphism in Drosophila mediopunctata: genetic (chromosomal) analysis and nonrandom association with chromosome inversions. Heredity 93:525–534
Hatadani LM, Klaczko LB (2008) Shape and size variation on the wing of Drosophila mediopunctata: influence of chromosome inversions and genotype-environment interaction. Genetica (The Hague), in press. DOI 10.1007/s10709-007-9217-7
Hoffmann AA, Shirriffs J (2002) Geographic variation for wing shape in Drosophila serrata. Evolution 56:1068–1073
Hosken DJ, Stockley P (2004) Sexual selection and genital evolution. Trends Ecol Evol 19:87–93
House CM, Simmons LW (2003) Genital morphology and fertilization success in the dung beetle Onthophagus taurus: an example of sexually selected male genitalia. Proc R Soc Lond B 270:447–455
House CM, Simmons LW (2005) The evolution of male genitalia: patterns of genetic variation and covariation in the genital sclerites of the dung beetle Onthophagus taurus. J Evol Biol 18:1281–1292
Imasheva AG, Moreteau B, David JR (2001) Growth temperature and genetic variability of wing dimensions in Drosophila: opposite trends in two sibling species. Genet Res Camb 76:237–247
Jennions MD, Kelly CD (2002) Geographical variation in male genitalia in Brachyrhaphis episcopi (Poeciliidae): is it sexually or naturally selected? Oikos 97:79–86
Kelly CD, Godin J-GJ, Abdallah G (2000) Geographic variation in the male intromittent organ of the Trinidadian guppy (Poecilia reticulata). Can J Zool 78:1674–1680
Klaczko LB (1995) Population genetics of D. mediopunctata. In: Levine LI (ed) Genetics of natural populations: the continuing importance of Theodosius Dobzhansky. Columbia Univ. Press, New York, pp 140–153
Klaczko LB (2006) Evolutionary genetics of Drosophila mediopunctata. Genetica 126:43–55
Kulikov AM, Melnikov AI, Gornostaev NG et al (2004) Morphological analysis of male mating organ in the Drosophila virilis species group: a multivariate approach. J Zool Syst Evol Res 42:135–144
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. The Auk 104:116–121
Lüpold S, Mcelligott AG, Hosken DJ (2004) Bat genitalia: allometry, variation and good genes. Biol J Linn Soc 83:497–507
Matta BP, Bitner-Mathé BC (2004) Genetic architecture of wing morphology in Drosophila simulans and an analysis of temperature effects on genetic parameter estimates. Heredity 93:330–341
Mayr E (1963) Animal species and evolution. Harvard University Press, Cambridge
Monteiro LR, Vinícius B, Reis SF (2005) Evolutionary integration and morphological diversification in complex morphological structures: mandible shape divergence in spiny rats (Rodentia, Echimyidae). Evol Dev 7:429–439
Moraes EM, Manfrin MH, Laus AC et al (2004) Wing shape heritability and morphological divergence of the sibling species Drosophila mercatorum and Drosophila paranaensis. Heredity 92:466–473
Mutanen M, Kaitala A, Mönkkönen M (2006) Genital variation within and between three closely related Euxoa moth species: testing the lock-and-key hypothesis. J Zool 268:109–119
Norry FM, Vilardi JC, Hasson E (1997) Correlations among size-related traits are affected by chromosome inversions in an adaptative polymorphism in Drosophila buzzatii. Heredity 79:585–590
Ohno S, Hoshizaki S, Ishikawa Y et al (2003) Allometry of male genitalia in a lepidopteran species, Ostrinia latipennis (Lepidoptera: Crambidae). Appl Ent Zool 38:313–319
Orengo DJ, Prevosti A (1999) Wing-size heritability in a natural population of Drosophila subobscura. Heredity 82:100–106
Peixoto AA, Klaczko LB (1991) Linkage disequilibrium analysis of chromosomal inversion polymorfisms of Drosophila. Genetics 129:773–777
Polihronakis M (2006) Morphometric analysis of intraspecific shape variation in male and female genitalia of Phyllophaga hirticula (Coleoptera: Scarabaeidae: Melolonthinae). Ann Ent Soc Am 99:144–150
Pretorius E, Scholtz CH (2001) Geometric morphometrics and the analysis of higher taxa: a case study based on the metendosternite of the Scarabaeoidea (Coleoptera). Biol J Linn Soc 74:35–50
Ramos M, Coddington JA, Christenson TE et al (2005) Have male and female genitalia coevolved? A phylogenetic analysis of genitalic morphology and sexual size dimorphism in web-building spiders (Araneae: Araneoidea). Evolution 59:1989–1999
Roff DA (1997) Evolutionary quantitative genetics. Chapman & Hall, New York
Rohlf FJ (1998) TpsDig, version 1.20. Department of Ecology and Evolution. State Univ. of New York at Stony Brook. Available at: http://life.bio.sunysb.edu/morph
Rohlf FJ (2003) TpsSmall, version 1.20. Department of Ecology and Evolution. State Univ. of New York at Stony Brook. Available at: http://life.bio.sunysb.edu/morph
Rohlf FJ (2004) TpsRelw, version 1.39. Department of Ecology and Evolution. State Univ. of New York at Stony Brook. Available at: http://life.bio.sunysb.edu/morph
Rohlf FJ, Bookstein F (2003) Computing the uniform component of shape variation. Syst Biol 52:66–69
Rohlf FJ, Slice DE (1990) Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Rowe L, Arnqvist G, Sih A et al (1994) Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol Evol 9:289–293
Scudder GGE (1971) Comparative morphology of insect genitalia. Annu Rev Ent 16:379–406
Shapiro AM, Porter AH (1989) The lock-and-key hypothesis evolutionary and biosystematic interpretation of insect genitalia. Ann Rev Ent 34:231–245
Sirot LK (2003) The evolution of insect mating structures through sexual selection. Florida Entomol 86:124–133
Slice DE (1996) Three-dimensional generalized resistant fitting and the comparison of least squares and resistant fit residuals. In: Marcus LF, Corti M, Loy A, Naylor G, Slice DE (eds) Advances in morphometrics. Plenum Press, New York, pp 179–200
Soto IM, Carreira VP, Fanara JJ et al (2007) Evolution of male genitalia: environmental and genetic factors affect genital morphology in two Drosophila sibling species and their hybrids. BMC Evol Biol 7:77–88
Thomas RH, Barker JSF (1993) Quantitative genetic analysis of the body size and shape of Drosophila buzzatii. Theor Appl Genet 85:598–608
Tidon-Sklorz R, Sene FM (1995) Evolution of the buzzatii cluster (Drosophila repleta species group) in middle South America. Evolución Biológica 8 y 9:71–85
Val FC, Vilela CR, Marques MD (1981) Drosophilidae of neotropical region. In: Ashburner M, Carson HC, Thompson JN Jr (eds) The genetics and biology of Drosophila, vol 3a. Academic Press, London
Vilela CR (1983) A revision of the Drosophila repleta species group (Diptera, Drosophilidae). Revta Bras Ent 27:1–114
Vilela CR, Bachli G (1990) Taxonomic studies on Neotropical species of seven genera of Drosophilidae (Diptera). Mitt Scheiz Ent Ges 63(Suppl.):1–332
Vilela RC, Pereira MAQP (1985) On the male genitalia of five species of the tripunctata group of Drosophila (Diptera, Drosophilidae). Rev Bras Ent 29:453–461
Wenninger EJ, Averill AL (2006) Influence of body and genital morphology on relative male fertilization success in oriental beetle. Behav Ecol 17:656–663
Wheeler MR, Kambysellis MP (1966) Notes on the Drosophilidae (Diptera) of Samoa. Univ Texas Publs 6615:533–565
Wilcockson RW, Crean CS, Day TH (1995) Heritability of a sexually selected character expressed in both sexes. Nature 374:158–159
Acknowledgements
It is a pleasure to thank Mary A. Heidi Dolder for her kind help with scanning electron micrographs; Wilma Nascimento de Souza for technical help and Ted Hogan for reviewing the English version of the manuscript. An anonymous reviewer made valuable comments on the manuscript. The administrators of Fundação José Pedro de Oliveira allowed us to collect at Mata Santa Genebra. This work was supported by Grants from: Programa Institucional de Capacitação Docente e Técnica (PICDT/Capes); Conselho Nacional de Desenvolvimento Técnico e Científico (CNPq); Fundação de Amparo à Pesquisa de São Paulo (FAPESP); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrade, C.A.C., Vieira, R.D., Ananina, G. et al. Evolution of the male genitalia: morphological variation of the aedeagi in a natural population of Drosophila mediopunctata . Genetica 135, 13–23 (2009). https://doi.org/10.1007/s10709-008-9247-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-008-9247-9