Abstract
Two different forces are thought to contribute to the rapid accumulation of hybrid male sterility that has been observed in many inter-specific crosses, namely the faster male and the dominance theories. For male heterogametic taxa, both faster male and dominance would work in the same direction to cause the rapid evolution of male sterility; however, for taxa lacking differentiated sex chromosomes only the faster male theory would explain the rapid evolution of male hybrid sterility. It is currently unknown what causes the faster evolution of male sterility, but increased sexual selection on males and the sensitivity of genes involved in male reproduction are two hypotheses that could explain the observation. Here, patterns of hybrid sterility in crosses of genetically divergent copepod populations are examined to test potential mechanisms of faster male evolution. The study species, Tigriopus californicus, lacks differentiated, hemizygous sex chromosomes and appears to have low levels of divergence caused by sexual selection acting upon males. Hybrid sterility does not accumulate more rapidly in males than females in these crosses suggesting that in this taxon male reproductive genes are not inherently more prone to disruption in hybrids.


Similar content being viewed by others
Abbreviations
- PM:
-
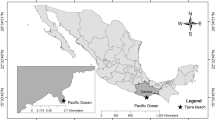
Punta Morro
- SD:
-
San Diego
- AB:
-
Abalone Cove
- SC:
-
Santa Cruz
- PES:
-
Pescadero
- PLA:
-
Playa Altamira
References
Ar-Rushdi AH (1963) The cytology of achiasmatic meiosis in the female Tigriopus (Copepoda). Chromosoma 13:526
Battaglia B (1982) Genetic variation and speciation events in marine copepods. In: Barigozzi C (eds) Mechanisms of speciation. Alan R Liss, New York, pp 377–392
Božić B (1960) Le genre Tigriopus Norman (Copépodes Harpacticoides) et ses formes Européennes, researches morphologiques et expérimentales. Arch Zool Exp Gen 98:167–269
Burton RS (1985) Mating system of the intertidal copepod Tigriopus californicus. Mar Biol 86:247–252
Burton RS (1987) Differentiation and integration of the genome in populations of Tigriopus californicus. Evolution 41:504–513
Burton RS (1990a) Hybrid breakdown in developmental time in the copepod Tigriopus californicus. Evolution 44:1814–1822
Burton RS (1990b) Hybrid breakdown in physiological response a mechanistic approach. Evolution 44:1806–1813
Burton RS (1998) Intraspecific phylogeography across the Point Conception biogeographic boundary. Evolution 52:734–745
Burton RS, Feldman MW (1981) Population genetics of Tigriopus californicus. II. Differentiation among neighboring populations. Evolution 35:1192–1205
Coyne JA, Orr HA (2004) Speciation. Sinauer Assoc., Sunderland, MA
Edmands S (1999) Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution 53:1757–1768
Edmands S (2001) Phylogeography of the intertidal copepod Tigriopus californicus reveals substantially reduced population differentiation at northern latitudes. Mol Ecol 10:1743–1750
Edmands S, Deimler JK (2004) Local adaptation, intrinsic coadaptation and the effects of environmental stress on interpopulation hybrids in the copepod Tigriopus californicus. J Experiment Marine Biol Ecol 303:183–196
Edmands S, Harrison JS (2003) Molecular and quantitative trait variation within and among populations of the intertidal copepod Tigriopus californicus. Evolution 57:2277–2285
Ellison CK, Burton RS (2006) Disruption of mitochondrial function in interpopulation hybrids of Tigriopus californicus. Evolution 60:1382–1391
Ganz HH, Burton RS (1995) Genetic differentiation and reproductive incompatibility among Baja California populations of the copepod Tigriopus californicus. Mar Biol 123:821–828
Haldane JBS (1922) Sex ratio and unisexual sterility in animal hybrids. J Genet 12:101–109
Hollocher H, Wu C-I (1996) The genetics of reproductive isolation in the Drosophila simulans clade: X versus autosomal effects and male vs. female effects. Genetics 143:1243–1255
Michalak P, Noor MAF (2003) Genome-wide patterns of expression in Drosophila pure species and hybrid males. Mol Biol Evol 20:1070–1076
Palmer CA, Edmands S (2000) Mate choice in the face of both inbreeding and outbreeding depression in the intertidal copepod Tigriopus californicus. Mar Biol 136:693–698
Presgraves DC (2002) Patterns of postzygotic isolation in Lepidoptera. Evolution 56:1168–1183
Presgraves DC, Orr HA (1998) Haldane’s rule in taxa lacking a hemizygous X. Science 282:952–954
Price T, Bouvier MM (2002) The evolution of F1 post-zygotic incompatibilities in birds. Evolution 56:2083–2089
Ranz JM, Namgyal K, Gibson G et al (2004) Anomalies in the expression profile of Interspecific hybrids of Drosophila melanogaster and Drosophila simulans. Genome Res 14:373–379
Rawson PD, Burton RS (2006) Molecular evolution at the cytochrome c oxidase subunit 2 gene among divergent populations of the intertidal copepod, Tigriopus californicus. J Mol Evol 62:753–764
Russell ST (2003) Evolution of intrinsic post-zygotic reproductive isolation in fish. Ann Zool Fennici 40:321–329
Sasa M, Chippendale PT, Johnson NA (1998) Patterns of postzygotic isolation in frogs. Evolution 52:1811–1820
Slotman M, della Torre A, Powell JR (2005) Female sterility in hybrids between Anopheles gambiae and A. arabiensis, and the causes of Haldane’s rule. Evolution 59:1016–1026
Tao Y, Chen S, Hartl DL et al (2003) Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the X and autosomes. Genetics 164:1383–1397
True JR, Weir BS, Laurie CC (1996) A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics 142:819–837
Turelli M, Moyle LC (2007) Asymmetric postmating isolation: Darwin’s corollary to Haldane’s rule. Genetics 176:1059–1088
Voorduow MJ, Anholt BR (2002a) Heritability of sex tendency in a harpacticoid copepod, Tigriopus californicus. Evolution 56:1754–1763
Voorduow MJ, Anholt BR (2002b) Environmental sex determination in a splash pool copepod. Biol J Linn Soc 76:511–520
Willett CS, Burton RS (2001) Viability of cytochrome c depends on cytoplasmic background in Tigriopus californicus. Evolution 55:1592–1599
Willett CS, Burton RS (2003) Environmental influences on epistatic interactions: viabilities of cytochrome c genotypes in interpopulation crosses. Evolution 57:2286–2292
Willett CS, Burton RS (2004) Evolution of interacting proteins in the mitochondrial electron transport system in a marine copepod. Mol Biol Evol 21:443–453
Wu C-I (1992) A note on Haldane’s rule: hybrid inviability versus hybrid sterility. Evolution 46:1584–1587
Wu C-I, Davis AW(1993) Evolution of post-mating reproductive isolation-the composite nature of Haldane’s rule and its genetic bases. Am Nat 142:187–212
Wu C-I, Johnson NA, Palopoli MF (1996) Haldane’s rule and its legacy: why are there so many sterile males? Trends Ecol Evol 11:281–284
Acknowledgements
I would like to thank J. Flowers, R. Burton, M. Turelli, and M. Servedio for helpful discussions on this project and M. Servedio and G. Wyngaard for comments on this manuscript. R. Byrne, R. Stuart, E. Hoddeson, Q. Qian, J. Berkowitz, E. Washburn helped with the copepod fertility experiments. C. W. was supported by money from UNC and National Science Foundation grant DEB-0516139.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willett, C.S. No evidence for faster male hybrid sterility in population crosses of an intertidal copepod (Tigriopus californicus). Genetica 133, 129–136 (2008). https://doi.org/10.1007/s10709-007-9191-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-007-9191-0