Abstract
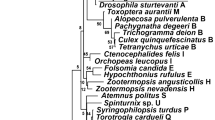
Wolbachia pipientis, an intracellular, α-proteobacterium, is commonly found in arthropods and filarial nematodes. Most infected insects are known to harbor strains of Wolbachia from supergroups A or B, whereas supergroups C and D occur only in filarial nematodes. Here, we present molecular evidence from two genes (ftsZ and 16S rDNA) that 2 Orthopterans (the bush cricket species Orocharis saltator and Hapithus agitator; Gryllidae: Eneopterinae) are infected with Wolbachia from the F supergroup. Additionally, a series of PCR tests revealed that these bush cricket specimens did not harbor nematodes, thus indicating that our positive results were not a by-product of nematodes being present in these cricket samples. Patterns of molecular variation suggest that (1) strains of F supergroup Wolbachia exhibit less genetic variation than the nematode-specific C and D supergroups but more than the A and B supergroups found in arthropods and (2) that there is no evidence of recombination within F supergroup strains. The above data support previous findings that F supergroup Wolbachia is not only harbored in both nematodes and arthropods, but that horizontal transfer has likely occurred recently between these diverse taxonomic groups (although the exact details of such horizontal transmissions remain unclear). Moreover, the limited genetic variation and lack of recombination in the F supergroup suggest that this clade of Wolbachia has radiated relatively rapidly with either (1) little time for recombination to occur or (2) selection against recombination as occurs in the mutualistic C and D strains of Wolbachia – both of which remain to be explored further.
Similar content being viewed by others
References
Baldo LS, Bordenstein S, Wernegreen JJ, Werren JH (2006) Widespread recombination throughout Wolbachia Genomes. Mol Bol Evol 23:437–449
Bandi C, Damiani G, Magrassi L, Grigolo A, Fani R, Sacchi L (1994) Flavobacteria as intracellular symbionts in cockroaches. Proc Roy Soc Lon B 257:43–48
Bandi C, Anderson TJC, Genchi C, Blaxter ML (1998) Phylogeny of Wolbachia in filarial nematodes. Proc R Soc Lond Ser B 265:2407–2413
Bandi C, McCall JW, Genchi C, Corona S, Venco L, Sacchi L (1999) Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int J Parasitol 29:357–364
Bandi C, Trees AJ, Brattig NW (2001) Wolbachia in Filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet Parasitol 98:215–238
Bordenstein SR, O’Hera FP, Werren JH (2001) Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409:707–710
Bordenstein SR, Rosengaus RB (2005) Discovery of a novel Wolbachia supergroup in Isoptera. Curr Microbiol 51:393–398
Boyle L, O’Neill SL, Robertson HM, Karr TL (1993) Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796–1799
Czarnetzki AB, Tebbe CC (2004) Detection and phylogenetic analysis of Wolbachia in Collembola. Environ Microbiol 6(1):35–44
Casiraghi M, Favia G, Cancrini G, Bartoloni A, Bandi C, 2001a. Molecular identification of Wolbachia from the filarial nematode Mansonella ozzardi. Parasitol. Res 87:417–420
Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C, 2001b. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitol 122:93–103
Casiraghi M, McCall JW, Simoncini L, Kramer LH, Sacchi L, Genchi C, Werren JH, Bandi C (2002) Tetracycline treatment and sex-ratio distortion: a role for Wolbachia in the moulting of filarial nematodes? Internat. J. Parasitol 32:1457–1468
Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua V, Gardner SL, Franceschi A, Bandi C (2004) Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Intern J Parasitol 34:191–203
Casiraghi M, Bordenstein SR, Baldo L, Lo N, Beninati T, Wernegreen JJ, Werren JH, Bandi C (2005) Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology 151:4015–4022
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hurst DD, Jiggins FM (2005) Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc Roy Soc Lond B 272:1525–1534
Jiggins FM (2002) The rate of recombination in Wolbachia bacteria. Mol Biol Evol 19:1640–1643
Jiggins FM, Hurst GDD, Yang Z (2002) Host-symbiont conflicts: positive selection on an outer membrane protein of parasitic but not mutualistic rickettsiaceae. Mol Biol Evol 19:1341–1349
Langworthy NG, Renz A, Mackenstedt U, Henkleduhrsen K, De Bronsvoort MB, Tanya VN, Donnelly MJ, Trees AJ (2000) Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc R Soc Lond B 267:1063–1069
Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C (2002) How many Wolbachia supergroups exist? Mol. Biol Evol 19:341–346
Marshall JL (2004) The Allonemobius-Wolbachia hostendosymbiont system: evidence for rapid speciation and against reproductive isolation driven by cytoplasmic incompatibility. Evolution 58:2409–2425
Martin D, Rybicki E (2000) RDP: detection of recombination amongst aligned sequences. Bioinformatics 16:562–563
Maynard Smith J (1992) Analyzing the mosaic structure of genes. J Mol Evol 34:126–129
Posada D (2002) Evaluation of methods for detecting recombination from DNA sequences: empirical data. Mol Biol Evol 19:708–717
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc Natl Acad Sci USA 98:13757–13762
Rasgon JL, Scott TW (2004) Phylogenetic characterization of Wolbachia symbionts infecting Cimex lectularius L. and Oeciacus vicarius Horvath. (Hemiptera: Cimicidae). J Med Entomol 41:1174–1178
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rozas J, Rozas R (1999) DnaSP version (3)0: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174–175
Rowley SM, Raven RJ, McGraw EA (2004) Wolbachia pipientis in Australian spiders. Curr Microbiol 49:208–214
Shoemaker DD, Katju V, Jaenike J (1999) Wolbachia and the evolution of reproductive isolation between Drosophila recens and Drosophila subquinaria Evolution 53:1157–1164
Swofford, DL (2002) PAUP, Phylogenetic Analysis using Parsimony (*and other methods):version 4. Sinauer Associates, Sunderland, Massachusetts
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 24:4876–4882
Vandekerckhove TMT, Watteyne S, Willems S, Swings JG, Mertens J, Gillis M (1999) Phylogenetic analysis of the 16s rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for Wolbachia taxonomy. FEMS Microbiol Lett 180:279–286
Weeks AR, Reynolds KT, Hoffmann AA (2002) Wolbachia dynamics and host effects: what has (and has not) been demonstrated? Trends Ecol. Evol 17:257–62
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42:587–609
Werren JH, Windsor DM (2000) Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc Lond B 261:1277–1285
Zhou WM, Rousset F, O’Neill S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B 265:509–515
Acknowledgements
We thank Drs. Claudio Bandi and Maurizio Casiraghi (Università di Milano) for providing the D. immitis sample. This work was funded by a grant from the Advanced Research Program of Texas to JLM (ARP 003656–0067–2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panaram, K., Marshall, J.L. F supergroup Wolbachia in bush crickets: what do patterns of sequence variation reveal about this supergroup and horizontal transfer between nematodes and arthropods?. Genetica 130, 53–60 (2007). https://doi.org/10.1007/s10709-006-0020-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-006-0020-7