Abstract
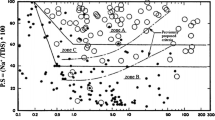
Soil dispersion is a phenomenon in which soil particles become afloat when they are exposed to water, and are carried away by the force of seepage. In spite of that soil dispersion is due to its chemical composition, the results obtained from the chemical methods, especially from the most widely used, Sherard method does not match with the results of well-known Pinhole test. This study tries to evaluate and modify the Sherard diagram for determination of dispersion potential of clayey soils. For this purpose, several natural soil samples were collected from different regions of Iran and some artificial soil samples were made by adding different percentages of four chemical agents, including sodium chloride, sodium carbonate, sodium sulfate, and sodium polyphosphate to a natural soil. The physical, chemical and index properties of all samples were determined and for determination of dispersion potential, the commonly used chemical test (Sherard method) and Pinhole test were employed. The results obtained from the tests showed that the Sherard chemical method which is solely based on the amount and type of the existing cations, is not able to determine soil dispersion correctly since the role of some anions, especially chloride is neglected. It was also found that among the existing anions in the soil, the chloride on the contrary to sodium acts as a flocculating factor. The results showed that by converting the vertical axis of the Sherard chart from sodium% to (sodium chloride)%, its conformity to the results of Pinhole tests increases considerably.



Similar content being viewed by others
References
Anon (2000) Annual book of ASTM standards. Vol 04.08, Soil and Rock, ASTM D420, D5779, D4221, D4647
Bhuvaneshwari S, Soundra B, Robinson RG, Gandhi SR (2007) Stabilization and microstructural modification of dispersive clayey soils. First international conference on soil and rock engineering organized by Srilankan Geotechnical Society, Columbo, Srilanka, 5–11 August 2007
Craft D, Acciardi RG (1984) Failure of pore water pressure analyses for dispersion. J Geotech Eng Div ASCE 110(4)
Didiek Djarwadi (2007) Dispersivity test of Duriangkang dam filling material dinamika. TEKNIK SIPIL 7(1):11–19
Elges HFWK (1985) Problem soils in South Africa-State of the Art. Civ Eng S Afr 27(7):347–349
Farzaneh O, Rezvan A, Bicheranloo Reza (2001) The effect of extraction moisture on dispersivity potential of sample using Sherard method. J Tech Coll Tehran Univ 35(4):513–522 (in Farsi)
Frenkel H, Levy GJ (1992) Clay dispersion and hydraulic conductivity of clay-sand mixtyres as affected by the addition of various anions. J Clay Clay Miner 40(5):515–521
Ingles OG, Aitchison GD (1969) Soil−water disequilibrium as a cause of subsidence in natural soils and earth embankments. In: symposium on land subsidence, Tokyo, AIHS Publication no 89, vol 2
Knodel PC (1991) Characteristica and problems of dispersive clay soils. US Department of the Interior, Bureau of Reclamation, Denver, Office, R-91-09
Lashkaripour GR, Khamehchiyan M, Soloki HR, Rahimi E (2006) The characteristics of dispersive soils in Sistan plain, eastern Iran. J Appl Geol 3(1):75–80
Ouhadi Vahid, Goodarzi R, Amir R (2006) Assessment of the stability of a dispersive soil treated by alum. J Eng Geol 85:91–101
Panayiotopoulosk P, Barbayiannis N, Papatolios K (2004) Influence of electrolyte concentration, sodium adsorption ratio, and mechanical disturbance on dispersed clay particle size and critical flocculation concentration in Alfisols. Commun Soil Sci Plant Anal 35(9–10):1415–1434
Rahimi H, Abbasi N (2008) Failure of concrete canal lining on fine sandy soils (A case study for Saveh Project). J Irrig Drainage 57:83–92
Rahimi H, Delfy M (1992) Recognition of dispersive clay in Khuzestan Province. M.Sc thesis, Department of irrigation and drainage, University of Tehran
Shanmuganathan RT, Oades JM (1983) Influence of anions on dispersion and physical properties of the horizon of a Red-brown earth. Geoderma 29(3):257–277
Sherard JL, Dunnigan LP, Decker RS (1976) Identification and natures of dispersive soils. J Geotech Eng Div 102(4):287–301
Sparks Donald (2000) Soil physical chemistry. CRC Press, Florida 33431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbasi, N., Nazifi, M.H. Assessment and Modification of Sherard Chemical Method for Evaluation of Dispersion Potential of Soils. Geotech Geol Eng 31, 337–346 (2013). https://doi.org/10.1007/s10706-012-9573-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-012-9573-7