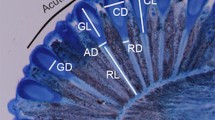
Abstract
Color changes and pattern formations can represent strategies of the utmost importance for the survival of individuals or of species. Previous studies have associated capture with the formation of blotches (areas with light color) of coral trout, but the regulatory mechanisms link the two are lacking. Here, we report that capture induced blotches formation within 4–5 seconds. The blotches disappeared after anesthesia dispersed the pigment cells and reappeared after electrical stimulation. Subsequently, combining immunofluorescence, transmission electron microscopy and chemical sympathectomy, we found blotches formation results from activation of catecholaminergic neurons below the pigment layer. Finally, the in vitro incubation and intraperitoneal injection of norepinephrine (NE) induced aggregation of chromatosomes and lightening of body color, respectively, suggesting that NE, a neurotransmitter released by catecholaminergic nerves, mediates blotches formation. Our results demonstrate that acute stress response-induced neuronal activity can drive rapid changes in body color, which enriches our knowledge of physiological adaptations in coral reef fish.







Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Amiri M (2009) Postsynaptic alpha 2-adrenoceptors mediate melanosome aggregation in melanophores of the white-spotted rabbitfish (Siganus canaliculatus). Pak J Biol Sci 12:1
Amiri M, Shaheen HM (2012) Chromatophores and color revelation in the blue variant of the Siamese fighting fish (Betta splendens). Micron 43:159–169
Andersson R, Karlsson J, Grundström N (1984) Adrenergic nerves and the alpha2-adrenoceptor system regulating melanosome aggregation within fish melanophores. Acta Physiol Scand 121(2):173–179. https://doi.org/10.1111/j.1748-1716.1984.tb07444.x
Archer SK, Heppell SA, Semmens BX, Pattengill-Semmens CV, Bush PG, Mccoy CM, Johnson BC (2012) Patterns of color phase indicate spawn timing at a Nassau grouper Epinephelus striatus spawning aggregation. Current Zoology 58:73–83
Aspengren S, Sköld HN, Quiroga G, Mårtensson L, Wallin M (2003a) Noradrenaline-and melatonin-mediated regulation of pigment aggregation in fish melanophores. Pigment Cell Res 16:59–64
Aspengren S, Sköld HN, Quiroga G, Mårtensson L, Wallin M (2003b) Noradrenaline-and melatonin-mediated regulation of pigment aggregation in fish melanophores. Pigment Cell Res 16(1):59–64. https://doi.org/10.1034/j.1600-0749.2003.00003.x
Ballowitz E (1893) Die Nervenendigungen der Pigmentzellen, ein Beitrag zur Kenntnis des Zusammenhanges der Endverzweigungen der Nerven mit dem Protoplasma der Zellen. Zeit. Wiss. Zool. 56:673–706
Bandaranayake WM (2006) The nature and role of pigments of marine invertebrates. Nat Prod Rep 23:223–255
Bauer DH, Demski LS (1980) Vertical banding evoked by electrical stimulation of the brain in anaesthetized green sunfish, Lepomis cyanellus, and bluegills, Lepomis macrochirus. J Exp Biol 84:149–160
Biswas SP, Jadhao AG, Palande NV (2014) Role of catecholamines and nitric oxide on pigment displacement of the chromatophores of freshwater snakehead teleost fish, Channa punctatus. Fish Physiol Biochem 40:457–467
Burton D (1985) Differential in vivo sensitivity of melanophores and xanthophores to catecholamines in winter flounder (Pseudopleuronectes americanus Walbaum) integumentary patterns. J Exp Biol 114:649–659
Burton D, Mayo D (1995) Adrenoceptors in cryptic patterning of a flatfish, Pleuronectes americanus. Proc R Soc London. Ser B: Biol Sci 261:181–186
Burton D, Vokey J (2000) α1-and α2-adrenoceptor mediation in melanosome aggregation in cryptic patterning of Pleuronectes americanus. Comp Biochem Physiol a: Mol Integr Physiol 125:359–365
Choi S-H, Kim B-H, Lee C-H, Lee Y-D (2020) Response of body color change rearing under different light intensity conditions in farmed red spotted grouper, Epinephelus akaara. Fish Aquat Sci 23:1–9
CL S (1961) Synopsis of biological data on groupers (Epinephelus and allied genera) of the Western North Atlantic. FAO Fish Biol. Synopses 23:61
Colin PL (1992) Reproduction of the Nassau grouper, Epinephelus striatus (Pisces: Serranidae) and its relationship to environmental conditions. Environ Biol Fishes 34:357–377
Cubukcu E, Kahraman I (2008) Hue, saturation, lightness, and building exterior preference: An empirical study in Turkey comparing architects’ and nonarchitects’ evaluative and cognitive judgments. Color Res Appl: Endorsed Inter-Soc Color Council, The Colour Group (Great Britain) Canadian Soc Color, Color Sci Assoc Japan, Dutch Soc Study of Color, Swedish Colour Centre Foundation, Colour Soc Australia, Centre Français De La Couleur 33:395–405
Cunha MA, Berglund A, Monteiro NM (2017) Female ornaments signal own and offspring quality in a sex-role-reversed fish with extreme male parental care. Marine Ecology 38(5):e12461. https://doi.org/10.1111/maec.12461
Deloach N (1993) Reef coral identification: Florida. Jacksonville, FL. New World Publications Inc, Caribbean, Bahamas
Demski L (1969) Behavioral effects of electrical stimulation of the brain in free-swimming bluegills (Lepomis macrochirus). University of Rochester, New York, USA, Ph.D. dissertation
Enami M (1955) Melanophore-contracting hormone (MCH) of possible hypothalamic origin in the catfish. Parasilurus Sci 121(3132):36–37. https://doi.org/10.1126/science.1121.3132.1136
Figon F, Casas J (2018) Morphological and physiological colour changes in the animal kingdom. eLS: 1–11. https://doi.org/10.1002/9780470015902.a9780470028065
Fujii R (2000) The regulation of motile activity in fish chromatophores. Pigment Cell Res 13(5):300–319. https://doi.org/10.1034/j.1600-0749.2000.130502.x
Fujii R, Oshima N (1986) Control of chromatophore movements in teleost fishes. Zoolog Sci 3(1):13–47
Fujii R, Oshima N (1994) Factors influencing motile activities of fish chromatophores. In: Arpigny JL, Coyette J, Davail S, Feller G, Fonzé E, Foulkes EC, Frère J-M, Fujii R, Génicot S, Gerday C, Joris B, Lamotte-Brasseur J, Maina JN, Narinx E, Nguyen-Disteche M, Oshima N, Viarengo A, Zekhnini Z (eds) Advances in Comparative and Environmental Physiology. Springer, Berlin, Heidelberg, pp 1–54. https://doi.org/10.1007/978-3-642-78598-6_1
Fujishige A, Moriwake T, Ono A, Ishii Y, Tsuchiya T (2000) Control of melanosome movement in intact and cultured melanophores in the bitterling, Acheilognathus lanceolatus. Comp Biochem Physiol A: Mol Integr Physiol 127:167–175
Glinka Y, Gassen M, Youdim M (1997) Mechanism of 6-hydroxydopamine neurotoxicity. in: Riederer P, Calne DB, Horowski R, Mizuno Y, Poewe W, Youdim MBH (Eds), Advances in Research on Neurodegeneration. J Neural Transm. Supplementa. Springer. Berlin, German, pp. 55-66. https://doi.org/10.1007/978-3-7091-6842-4_7
Goda M, Kuriyama T (2021) Physiological and Morphological Color Changes in Teleosts and in Reptiles. In: Hashimoto H, Goda M, Futahashi R, Kelsh R, Akiyama T (eds) Pigments, Pigment Cells and Pigment Patterns. Springer, Singapore, pp 387–423. https://doi.org/10.1007/978-981-16-1490-3_13
Hanlon R, Chiao C-C, Mäthger L, Barbosa A, Buresch K, Chubb C (2009) Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Phil Trans R Soc B: Biol Sci 364:429–437
Heemstra PC, Randall JE (1993) FAO species catalogue Vol. 16. Groupers of the world (family: Serranidae, Subfamily: Epinephelinae). An annotated and illustrated catalogue of the grouper, rocked, hind, coral grouper and lyretail species known to date. FAO Fisheries synopsis: FAO, Rome, p. 382
Jones BE, Beaudet A (1987) Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol 261(1):15–32. https://doi.org/10.1002/cne.902610103
Junqueira L, Salles L (1978) Effects of 6-hydroxydopamine and 5-hydroxydopamine onlthe teleost melanophores innervation. J Fish Biol 13(4):415–419. https://doi.org/10.1111/j.1095-8649.1978.tb03449.x
Kasukawa H, Oshima N (1987) Divisionistic generation of skin hue and the change of shade in the scalycheek damselfish, Pomacentrus lepidogenys. Pigment Cell Res 1:152–157
Kasukawa H, Sugimoto M, Oshima N, Fujii R (1985) Control of chromatophore movements in dermal chromatic units of blue damselfish–I. The melanophore. Comparative biochemistry and physiology. C, Comp Pharmacol Toxicol 81:253–257
Kindermann C, Hero J-M (2016) Pigment cell distribution in a rapid colour changing amphibian (Litoria wilcoxii). Zoomorphology 135(2):197–203. https://doi.org/10.1007/s00435-016-0303-1
Kindermann C, Narayan EJ, Hero J-M (2014) The neuro-hormonal control of rapid dynamic skin colour change in an amphibian during amplexus. PLoS One 9(12):e114120. https://doi.org/10.1371/journal.pone.0114120
Kostrzewa RM, Jacobowitz DM (1974) Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev 26(3):199–288
Kumazawa T, Ryozo F (1984) Concurrent releases of norepinephrine and purines by potassium from adrenergic melanosome-aggregating nerve in tilapia. Comp Biochem Physiol Part C: Comp Pharmacol 78(2):263–266. https://doi.org/10.1016/0742-8413(84)90080-X
Ligon RA, McCartney KL (2016) Biochemical regulation of pigment motility in vertebrate chromatophores: a review of physiological color change mechanisms. Curr Zool 62(3):237–252. https://doi.org/10.1093/cz/zow051
Longley W (1917) Studies upon the biological significance of animal coloration. I. The colors and color changes of West Indian reef-fishes. J Exp Zool 23:533–601
Mårtensson LG, Andersson RG (2000) Is Ca2+ the second messenger in the response to melatonin in cuckoo wrasse melanophores? Life Sci 66:1003–1010
Maeno N, Iga T (1992) Adrenergic mechanisms associated with the movement of platelets in iridophores from the freshwater goby, Odontobutis obscura. Comp Biochem Physiol C, Comp Pharmacol Toxicol 102(2):233–237. https://doi.org/10.1016/0742-8413(92)90106-h
Marshall NJ, Cortesi F, de Busserolles F, Siebeck UE, Cheney KL (2019) Colours and colour vision in reef fishes: Past, present and future research directions. J Fish Biol 95:5–38
Masazumi S, Noriko O, Ryozo F (1985) Mechanisms controlling motile responses of amelanotic melanophores in the medaka, Oryzias latipes. Zoolog Sci 2:317–322
Nagai M, Oshima N, Fujii R (1986) A comparative study of melanin-concentrating hormone (MCH) action on teleost melanophores. Biol Bull 171(2):360–370. https://doi.org/10.2307/1541678
Nardocci G, Navarro C, Cortés PP, Imarai M, Montoya M, Valenzuela B, Jara P, Acuña-Castillo C, Fernández R (2014) Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol 40(2):531–538. https://doi.org/10.1016/j.fsi.2014.08.001
Nemtzov SC, Kajiura SM, Lompart CA (1993) Diel color phase changes in the coney, Epinephelus fulvus (Teleostei, Serranidae). Copeia 1993:883–885
Nguyen N, Sugimoto M, Zhu Y (2006) Production and purification of recombinant somatolactin β and its effects on melanosome aggregation in zebrafish. Gen Comp Endocrinol 145:182–187
Nilsson H, Rutberg M, Wallin M (1996) Localization of kinesin and cytoplasmic dynein in cultured melanophores from Atlantic cod, Gadus morhua. Cell Motil Cytoskelet 33:183–196
Oshima N, Kasukawa H, Fujii R (1989) Control of chromatophore movements in the blue-green damselfish, Chromis viridis. Comp Biochem Physiol Part C: Comp Pharmacol 93:239–245
Oshima N, Nakamaru N, Araki S, Sugimoto M (2001) Comparative analyses of the pigment-aggregating and-dispersing actions of MCH on fish chromatophores. Comp Biochem Physiol C: Toxicol Pharmacol 129:75–84
Qu M, Ding S, Xu X, Shen M, You Y, Su Y (2012) Ontogenetic development of the digestive system and growth in coral trout (Plectropomus leopardus). Aquaculture 334–337:132–141. https://doi.org/10.1016/j.aquaculture.2011.12.046
Ramachandran V, Tyler C, Gregory R, Rogers-Ramachandran D, Duensing S, Pillsbury C, Ramachandran C (1996) Rapid adaptive camouflage in tropical flounders. Nature 379:815–818
Ramlochansingh C, Branoner F, Chagnaud BP, Straka H (2014) Efficacy of tricaine methanesulfonate (MS-222) as an anesthetic agent for blocking sensory-motor responses in Xenopus laevis tadpoles. PloS One 9(7):e101606. https://doi.org/10.1371/journal.pone.0101606
Rudh A, Qvarnström A (2013) Adaptive colouration in amphibians, Seminars in cell & developmental biology. Elsevier, pp 553–561
Ryan RW, Post JI, Solc M, Hodson PV, Ross GM (2002) Catecholaminergic neuronal degeneration in rainbow trout assessed by skin color change: a model system for identification of environmental risk factors. Neurotoxicology 23(4–5):545–551. https://doi.org/10.1016/S0161-813X(02)00063-3
Sadovy Y, Eklund A-M (1999) Synopsis of biological data on the Nassau grouper, Epinephelus striatus (Bloch, 1792), and the jewfish, E. itajara (Lichtenstein, 1822). US Department Commer. National Oceanic and Atmospheric Administration Technical Report. National Marine Fisheries Service 146, and FAO Fisheries Synopsis 157, Seattle, Washington, p 65
Shimose T, Kanaiwa M (2022) Influence of the body color and size on the market value of wild captured coralgroupers (Serranidae, Plectropomus): Implications for fisheries management. Fish Res 248. https://doi.org/10.1016/j.fishres.2021.106223
Shinohara Y, Kasagi S, Amiya N, Hoshino Y, Ishii R, Hyodo N, Yamaguchi H, Sato S, Amano M, Takahashi A (2022) Taisho-Sanshoku koi have hardly faded skin and show attenuated melanophore sensitivity to adrenaline and melanin-concentrating hormone. Front Endocrinol 13:994060
Shwartz Y, Gonzalez-Celeiro M, Chen C-L, Pasolli HA, Sheu S-H, Fan SM-Y, Shamsi F, Assaad S, Lin ET-Y, Zhang B (2020) Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182(578–593):e519
Sköld HN, Norström E, Wallin M (2002) Regulatory control of both microtubule-and actin-dependent fish melanosome movement. Pigment Cell Res 15:357–366
Sköld HN, Yngsell D, Mubashishir M, Wallin M (2015) Hormonal regulation of colour change in eyes of a cryptic fish. Biology Open 4:206–211
Sköld HN, Aspengren S, Cheney KL, Wallin M (2016) Fish chromatophores—from molecular motors to animal behavior. Int Rev Cell Mol Biol 321:171–219
Sköld HN, Amundsen T, Svensson PA, Mayer I, Bjelvenmark J, Forsgren E (2008) Hormonal regulation of female nuptial coloration in a fish. Horm Behav 54:549–556
Smith RI (1942) Nervous control of chromatophores in the leech Placobdella parasitica. Physiol Zool 15(4):410–417. https://doi.org/10.2307/30151654
Sonka M, Hlavac V, Boyle R (2013) Image processing, analysis and machine vision. Springer
Stevens M, Merilaita S (2011) Animal camouflage: mechanisms and function. Cambridge University Press
Svensson S, Karlsson J, Grundström N (1989) Melanophores in isolated scales of Labrus berggylta (ascanius): innervation and alpha2-adrenoceptor-mediated pigment aggregation. Comp Biochem Physiol Part C: Comp Pharmacol 93:247–251
Swierk L (2022) Color change, encyclopedia of animal cognition and behavior. Springer, pp 1522–1525
Townsend CH (1909) Observations on instantaneous changes in color among tropical fishes. Kalkhoff Company
Townsend CH (1929) Records of changes in color among fishes. Zoologica 9:321–378
Uchida-oka N, Sugimoto M (2001) Norepinephrine induces apoptosis in skin melanophores by attenuating cAMP-PKA Signals Via α2-Adrenoceptors in the Medaka, Oryzias Latipes. Pigment Cell Res 14:356–361
Urban J, Štys D, Sergejevová M, Masojídek J (2013) Expertomica Fishgui: comparison of fish skin colour. J Appl Ichthyol 29:172–180
Watson A, Siemann L, Hanlon R (2014) Dynamic camouflage by Nassau groupers Epinephelus striatus on a Caribbean coral reef. J Fish Biol 85:1634–1649
Yamada K, Miyata S, Katayama H (1984) Autoradiographic demonstration of adrenergic innervation to scale melanophores of a teleost fish, Oryzias latipes. J Exp Zool 229:73–80
Yamaguchi D, Kasagi S, Shimizu D, Maeda T, Takahashi A, Mizusawa K (2022) A low body-color regulating ability of spotted halibut Verasper variegatus: Evaluation of the roles of melanin-concentrating hormone and proopiomelanocortin systems. Fish Sci 88(3):411–418. https://doi.org/10.1007/s12562-022-01600-6
Yasir I, Qin JG (2009) Effect of light intensity on color performance of false clownfish, Amphiprion ocellaris Cuvier. J World Aquac Soc 40:337–350
Zhang B (2020) Hyperactivation of sympathetic nerves drives melanocyte stem cell depletion. Nature 577:676–681. https://doi.org/10.1038/s41586-020-1935-3
Zhou K, Xin Wen, Cheng Deng, Shuailong Chen, Xingzhu Qi, Jian L (2021) Analysis of pigment cells difference in body color variation of Plectropomus leopardus. Acta Hydrobiologica Sinica 45(5):1164–1170. https://doi.org/10.7541/2021.2020.1291
Acknowledgements
We thank technician Xinming Chen of Xiao Deng Fisheries Technology Co., LTD for his help in obtaining and transporting experimental fish. We appreciate Chenchen Shi (PhD candidate) of the College of Ocean and Earth sciences, Xiamen University for her assistance in collecting samples for ultrastructure observation.
Funding
This work was supported by the Key Project of Agriculture in Fujian Province of China (No. 2021N0001, 2021NZ033016).
Author information
Authors and Affiliations
Contributions
Study conception and design: Nannan Zhao, Shi Xi Chen; material preparation, data collection and analysis: Nannan Zhao, Ke Jiang, Xiaoyu Ge, Jing Huang; methodology: Nannan Zhao, Caiming Wu; data curation: Nannan Zhao; writing — original draft: Nannan Zhao; writing — review and editing: Shi Xi Chen; Resources: Shi Xi Chen; project administration: Shi Xi Chen. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The Laboratory Animal Management and Ethics Committee of Xiamen University gave its approval to all animal experiments, which were planned to reduce pain and the number of animals utilized.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10695_2024_1300_MOESM1_ESM.pdf
Supplementary file1. Fig. S1 The electrical stimulation device and stimulation process. (A) The JL-A electronic stimulator; (B) Electrode connected to electrical stimulation devices; (C-D) Stimulation site and skin color before and after electrical stimulation (black circle) (PDF 85 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, N., Jiang, K., Ge, X. et al. Neurotransmitter norepinephrine regulates chromatosomes aggregation and the formation of blotches in coral trout Plectropomus leopardus. Fish Physiol Biochem 50, 705–719 (2024). https://doi.org/10.1007/s10695-024-01300-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-024-01300-1