Abstract
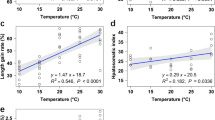
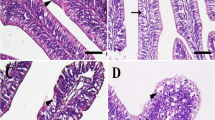
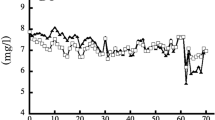
The present study aimed to investigate the effect of thermal stress on growth, feed utilization, coloration, hematology, liver histology, and critical thermal maximum (CTmax) in goldfish (Carassius auratus) cultured at three different acclimation temperatures including 27 °C, 30 °C, and 34 °C for 10 weeks. Goldfish were assigned randomly to tanks with a quadruplicate setup, accommodating 20 fish per tank. The result showed that fish acclimated to different temperatures did not significantly differ in weight gain (WG) and specific growth rate (SGR). However, increasing temperature significantly decreased feed efficiency ratio (FER), protein efficiency ratio (PER), and protein productive value (PPV), but significantly increased feed conversion ratio (FCR) (P < 0.05). The coloration parameters significantly decreased by high temperature in the trunk region with increasing temperature (L* and a* at week 5; L*, a*, and b* at week 10; P < 0.05). Total carotenoid contents in serum, fin, muscle, and skin also significantly decreased with increasing temperature (P < 0.05). Total protein, albumin, and globulin levels exhibited a notable decrease, while the albumin: globulin ratio showed a slight insignificant increase, with increasing temperature. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total cholesterol, and triglycerides significantly increased with increasing temperature (P < 0.05). While, high-density lipoprotein cholesterol (HDL-c) decreased linearly (P < 0.05). Glucose and cortisol levels linearly increased with increasing temperature, the highest levels being observed in the 34 °C group. Liver histology showed swollen hepatocytes, nuclei displacement, and infiltration of inflammation in fish cultured at 34 °C. Goldfish acclimated to 34 °C displayed a higher CTmax of 43.83 °C compared to other groups. The present study showed that temperature should be kept below 34 °C for goldfish culture to prevent high FCR, fading coloration, and liver damages.



Similar content being viewed by others
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Ahmed I, Reshi QM, Fazio F (2020) The influence of the endogenous and exogenous factors on hematological parameters in different fish species: a review. Aquac Int 28:869–899. https://doi.org/10.1007/s10499-019-00501-3
Alfonso S, Gesto M, Sadoul B (2021) Temperature increase and its effects on fish stress physiology in the context of global warming. J Fish Biol 98(6):1496–1508. https://doi.org/10.1111/jfb.14599
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists International, Arlington, VA, USA
Ashaf-Ud-Doulah M, Shahjahan Md, Islam SMM, Emran MA, Rahman MS, Hossain MAR (2019) Thermal stress causes nuclear and cellular abnormalities of peripheral erythrocytes in Indian major carp, rohu Labeo rohita. J Therm Biol 86:102450. https://doi.org/10.1016/j.jtherbio.2019.102450
Ashaf-Ud-Doulah M, Mamun AA, Rahman ML, Islam SMM, Jannat R, Hossain MAR, Shahjahan M (2020) High temperature acclimation alters upper thermal limits and growth performance of Indian major carp, rohu, Labeo rohita (Hamilton, 1822). J Therm Biol 93:102738. https://doi.org/10.1016/j.jtherbio.2020.102738
Babin A, Saciat C, Teixeira M, Troussard JP, Motreuil S, Moreau J, Moret Y (2015) Limiting immunopathology: interaction between carotenoids and enzymatic antioxidant defences. Dev Comp Immunol 49(2):278–281. https://doi.org/10.1016/j.dci.2014.12.007
Backström T, Brännäs E, Nilsson J, Magnhagen C (2014) Behaviour, physiology and carotenoid pigmentation in Arctic charr Salvelinus alpinus. J Fish Biol 84:1–9. https://doi.org/10.1111/jfb.12240
Balm PHM, Lambert JDG, Wendelaar Bonga SE (1989) Corticosteroid biosynthesis in the interrenal cells of the teleost fish. Oreochromis Mossambicus. Gen Comp Endocr 76(53):62
Barbosa MJ, Morais R, Choubert G (1999) Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture 176(3):331–341. https://doi.org/10.1016/S0044-8486(99)00115-5
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42(3):517–525. https://doi.org/10.1093/icb/42.3.517
Beach RH, Viator CL (2008) The economics of aquaculture insurance: an overview of the U.S. pilot insurance program for cultivated clams. Aquacult Econ Manag 12:25–38
Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of north American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58(3):237–275. https://doi.org/10.1023/A:1007676325825
Bergot F, Breque J (1983) Digestibility of starch by rainbow trout: effects of the physical state of starch and of the intake level. Aquaculture 34(3):203–212. https://doi.org/10.1016/0044-8486(83)90203-X
Blanco AM, Sundarrajan L, Bertucci JI, Unniappan S (2018) Why goldfish? Merits and challenges in employing goldfish as a model organism in comparative endocrinology research. Gen Comp Endocrinol 257:13–28
Brown J, Walker S, Steinman K (2004) Endocrine manual for the reproductive assessment of domestic and non-domestic species. Endocrine Research Laboratory, Department of Reproductive Sciences, Conservation and Research Center, National Zoological Park, Smithsonian Institution, Handbook. 1–93
Cai LS, Wang L, Song K, Lu K-L, Zhang CX, Rahimnejad S (2020a) Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 516:734615. https://doi.org/10.1016/j.aquaculture.2019.734615
Cai X, Zhang J, Lin L, Li Y, Liu X, Wang Z (2020b) Study of a noninvasive detection method for the high-temperature stress response of the large yellow croaker (Larimichthys crocea). Aquac Rep 18:100514. https://doi.org/10.1016/j.aqrep.2020.100514
Chatterjee N, Pal AK, Das T, Mohammed MS, Sarma K, Venkateshwarlu G, Mukherjee SC (2006) Secondary stress responses in Indian major carps Labeo rohita (Hamilton), Catla catla (Hamilton) and Cirrhinus mrigala (Hamilton) fry to increasing packing densities. Aquac Res 37(5):472–476. https://doi.org/10.1111/j.1365-2109.2006.01469.x
CIE (1976) Recommendations on uniform color spaces, color difference equations, psychometric color terms. Supplement No. 2 to CIE Publication No 15, Colorimetry. Bureau Central de la CIE, Paris, International commission on illumination
Collins M, Sutherland M, Bouwer L, Cheong S-M, FrölicherT, Jacot Des Combes H, Koll Roxy M, Losada I, McInnes K, Ratter B, Rivera-Arriaga E, Susanto RD, Swingedouw D, Tibig L (2019) Extremes, abrupt changes and managing risk. In: Pörtner H-O, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds) IPCC special report on the ocean and cryosphere in a changing climate, pp 589–674. https://www.ipcc.ch/srocc/cite-report/
Cossins AR, Friedlander MJ, Prosser CL (1997) Correlations between behavioral temperature adaptaions of goldfish and viscosity and fatty acid composition of their synaptic membrances. J Comp Physio 120:109–121
Coz-Rakovac R, Smuc T, Topic Popovic N, Strunjak-Perovic I, Hacmanjek M, Jadan M (2008) Novel methods for assessing fish blood biochemical data. J Appl Ichthyol 24(1):77–80. https://doi.org/10.1111/j.1439-0426.2007.01041.x
Dalvi RS, Pal AK, Tiwari LR, Das T, Baruah K (2009) Thermal tolerance and oxygen consumption rates of the catfish Horabagrus brachysoma (Günther) acclimated to different temperatures. Aquaculture 295(1):116–119. https://doi.org/10.1016/j.aquaculture.2009.06.034
Das T, Pal AK, Chakraborty SK, Manush SM, Sahu NP, Mukherjee SC (2005) Thermal tolerance, growth and oxygen consumption of Labeo rohita fry (Hamilton, 1822) acclimated to four temperatures. J Therm Biol 30(5):378–383. https://doi.org/10.1016/j.jtherbio.2005.03.001
Dharmaraj S, Dhevendaran K (2011) Application of microbial carotenoids as a source of colouration and growth of ornamental fish Xiphophorus helleri. WJFMS 137:137–144
Drupt F, Paris M, Frydman A, Leclerc M (1974) Serum albumin assay by bromocresol green method: application to different automatic apparatus. Ann Pharm Fr 32(5):249–256
Eslamloo K, Akhavan SR, Eslamifar A, Henry MA (2015) Effects of background colour on growth performance, skin pigmentation, physiological condition and innate immune responses of goldfish Carassius Auratus. Aquac Res 46(1):202–215. https://doi.org/10.1111/are.12177
FAO (2021) 3rd International Ornamental Fish Trade and Technical Conference. https://www.fao.org/in-action/globefish/news-events/details-news/en/c/1373555/
Farag MR, Alagawany M, Khalil SR, El-Aziz RM, Zaglool AW, Moselhy AAA, Abou-Zeid SM (2022) Effect of parsley essential oil on digestive enzymes, intestinal morphometry, blood chemistry and stress-related genes in liver of Nile tilapia fish exposed to Bifenthrin. Aquaculture 546:737322. https://doi.org/10.1016/j.aquaculture.2021.737322
Ford T, Beitinger TL (2005) Temperature tolerance in the goldfish Carassius auratus. J Therm Biol 30(2):147–152. https://doi.org/10.1016/j.jtherbio.2004.09.004
Freshwater Ornamental Fish (2021) Exporter and Importers, Trade by country 2021. Available at: https://www.oec.world/en. Accessed 29 May 2023
Frölicher TL, Fischer EM, Gruber N (2018) Marine heatwaves under global warming. Nature 560(7718):360–364
Fry FEJ, Hart JS (1948) The relation of temperature to oxygen consumption in the goldfish. Biol Bull 94(1):66–77. https://doi.org/10.2307/1538211
Fu KK, Fu C, Qin YL, Bai Y, Fu SJ (2018) The thermal acclimation rate varied among physiological functions and temperature regimes in a common cyprinid fish. Aquaculture 495:393–401. https://doi.org/10.1016/j.aquaculture.2018.06.015
Gouveia L, Rema P (2005) Effect of microalgal biomass concentration and temperature on ornamental goldfish (Carassius auratus) skin pigmentation. Aquac Nutr 11(1):19–23. https://doi.org/10.1111/j.1365-2095.2004.00319.x
Gracey AY, Fraser EJ, Li W, Fang Y, Taylor RR, Rogers J, Brass A, Cossins AR (2004) Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc Natl Acad Sciences 101(48):16970–16975. https://doi.org/10.1073/pnas.0403627101
Ha BS, Kang DS, Kim JH, Choi OS, Ryu HY (1993) Metabolism of dietary carotenoids and effects to improve the body color of cultured founder and red sea bream. Bull Korean Fish Soc 26:91–101
Hall DM, Xu L, Drake VJ, Oberley LW, Oberley TD, Moseley PL, Kregel KC (2000) Aging reduces adaptive capacity and stress protein expression in the liver after heat stress. J Appl Physio 89(2):749–759. https://doi.org/10.1152/jappl.2000.89.2.749
Hassaan MS, El Nagar AG, Salim HS, Fitzsimmons K, El-Haroun ER (2019) Nutritional mitigation of winter thermal stress in Nile tilapia by propolis-extract: associated indicators of nutritional status, physiological responses and transcriptional response of delta-9-desaturase gene. Aquaculture 511:734256. https://doi.org/10.1016/j.aquaculture.2019.734256
Hesser EF (1960) Methods for routine fish hematology. Progress Fish-Culturist 22(4):164–171. https://doi.org/10.1577/1548-8659(1960)22[164:MFRFH]2.0.CO;2
Hrubec TC, Robertson JL, Smith SA (1997) Effects of temperature on hematologic and serum biochemical profiles of hybrid striped bass (Morone chrysops x Morone saxatilis). Am J Vet Res 58(2):126–130
Hunter RS, Harold RW (1987) The measurement of appearance, the, 2nd edn. John Wiley & Sons, New York, NY, p 411
IPPC (2014) Climate change 2014: Impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change, Bonn, Germany. https://www.ipcc.ch/report/ar5/wg2/
Islam MA, Uddin MH, Uddin MJ, Shahjahan M (2019) Temperature changes influenced the growth performance and physiological functions of Thai pangas Pangasianodon hypophthalmus. Aquac Rep 13:100179. https://doi.org/10.1016/j.aqrep.2019.100179
Jeamsripong S, Chuanchuen R, Atwill ER (2018) Assessment of bacterial accumulation and environmental factors in sentinel oysters and estuarine water quality from the Phang Nga estuary area in Thailand. Int J Environ Res Public Health 15(9):1970
Jobling M (1994) Ingestion, absorption and excretion. In: Jobling M (ed) Fish Bioenergetics. Chapman Hall, London, pp 99–119
Jung SJ, Kim NN, Choi YJ, Choi Y, Heo YS, Choi CY (2016) Effects of melatonin and green-wavelength LED light on the physiological stress and immunity of goldfish, Carassius auratus, exposed to high water temperature. Fish Physiol Biochem 42:1335–1346. https://doi.org/10.1007/s10695-016-0221-7
Katersky RS, Carter CG (2007) A preliminary study on growth and protein synthesis of juvenile barramundi, Lates calcarifer at different temperatures. Aquaculture 267(1–4):157–164
Kestemont P (1995) Influence of feed supply, temperature and body size on the growth of goldfish Carassius auratus larvae. Aquaculture 136(3):341–349. https://doi.org/10.1016/0044-8486(95)00060-7
Khieokhajonkhet A, Muichanta S, Aeksiri N, Ruttarattanamongkol K, Rojtinnakorn J, Kaneko G (2021) Evaluation of sacha inchi meal as a novel alternative plant protein ingredient for red hybrid tilapia (Oreochromis niloticus×O. mossambicus): growth performance, feed utilization, blood biochemistry, and histological changes. Anim Feed Sci Tech 278:115004. https://doi.org/10.1016/j.anifeedsci.2021.115004
Khieokhajonkhet A, Aeksiri N, Rojtinnakorn J, Van Doan H, Kaneko G (2022a) Sacha inchi meal as a fish-meal replacer in red hybrid tilapia (Oreochromis niloticus × O. mossambicus) feeds: effects on dietary digestibility, growth metrics, hematology, and liver and intestinal histology. Aquac Int 30:677–698. https://doi.org/10.1007/s10499-022-00833-7
Khieokhajonkhet A, Sangphrom S, Aeksiri N, Tatsapong P, Wuthijaree K, Kaneko G (2022b) Effects of long-term exposure to high temperature on growth performance, chemical composition, hematological and histological changes, and physiological responses in hybrid catfish [♂Clarias gariepinus (Burchell, 1822) ×♀C. macrocephalus. J Therm Biol 105:103226. https://doi.org/10.1016/j.jtherbio.2022.103226
Khieokhajonkhet A, Uanlam P, Ruttarattanamongkol K, Aeksiri N, Tatsapong P, Kaneko G (2022) Replacement of fish meal by black soldier fly larvae meal in diet for goldfish Carassius auratus: growth performance, hematology, histology, total carotenoids, and coloration. Aquaculture 561:738618. https://doi.org/10.1016/j.aquaculture.2022.738618
Khosravi-Katuli K, Mohammadi Y, Ranjbaran M, Ghanaatian H, Khazaali A, Paknejad H, Santander J (2021) Effects of mannan oligosaccharide and synbiotic supplementation on growth performance and immune response of gilthead sea bream (Sparus aurata) before and after thermal stress. Aquac Res 52:3745–3756. https://doi.org/10.1111/are.15220
Knox WE, Greengard O (1965) The regulation of some enzymes of nitrogen metabolism - an introduction to enzyme physiology. Adv Enzyme Regul 3:247–313. https://doi.org/10.1016/0065-2571(65)90059-2
Kumar V, Sahu NP, Pal AK, Kumar S (2007) Immunomodulation of Labeo rohita juveniles due to dietary gelatinized and non-gelatinized starch. Fish Shellfish Immunol 23(2):341–353. https://doi.org/10.1016/j.fsi.2006.11.008
Kumar P, Pal AK, Sahu NP, Jha AK, Kumar N, Christina L, Priya P (2018) Dietary L-Tryptophan potentiates non-specific immunity in Labeo rohita fingerlings reared under elevated temperature. J Therm Biol 74:55–62. https://doi.org/10.1016/j.jtherbio.2018.03.010
Li M, Wang X, Qi C, Li E, Du Z, Qin JG, Chen L (2018) Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 495:187–195. https://doi.org/10.1016/j.aquaculture.2018.05.031
Li B, Sun S, Zhu J, Yanli S, Wuxiao Z, Ge X (2019a) Transcriptome profiling and histology changes in juvenile blunt snout bream (Megalobrama amblycephala) liver tissue in response to acute thermal stress. Genomics 111(3):242–250. https://doi.org/10.1016/j.ygeno.2018.11.011
Li C, Wang Y, Wang G, Chen Y, Guo J, Pan C, Liu E, Ling Q (2019b) Physicochemical changes in liver and Hsc70 expression in pikeperch Sander lucioperca under heat stress. Ecotoxicol Environ Saf 181:130–137. https://doi.org/10.1016/j.ecoenv.2019.05.083
Liu B, Xu P, Brown PB, Xie J, Ge X, Miao L, Zhou Q, Ren M, Pan L (2016) The effect of hyperthermia on liver histology, oxidative stress and disease resistance of the Wuchang bream, Megalobrama amblycephala. Fish Shellfish Immunol 52:317–324. https://doi.org/10.1016/j.fsi.2016.03.018
Liu ZP, Gu WB, Tu DD, Zhu QH, Zhou YL, Wang C, Wang LZ, Shu MA (2018) Effects of both cold and heat stress on the liver of the giant spiny frog (Quasipaa spinosa): stress response and histological changes. J Exp Biol 221(21):186379. https://doi.org/10.1242/jeb.186379
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comp Biochem Physiol Part-c: Toxicol Pharmacol 143:36–41
Mambrini M, Kaushik SJ (1995) Indispensable amino acid requirements of fish: correspondence between quantitative data and amino acid profile of tissue proteins. J Appl Ichthyol 11:240–247. https://doi.org/10.1016/j.cbpc.2005.11.018
Mangi SC, Lee J, Pinnegar JK, Law R, Tyllianakis E, Birchenough SNR (2018) The economic impacts of ocean acidification on shellfish fisheries and aquaculture in the United Kingdom. Environ Sci Policy 86:95–105. https://doi.org/10.1016/j.envsci.2018.05.008
McDonnell LH, Chapman LJ (2015) At the edge of the thermal window: effects of elevated temperature on the resting metabolism, hypoxia tolerance and upper critical thermal limit of a widespread African cichlid. Conserv Physiol 3:1–13
Moreira IS, Peres H, Couto A, Enes P, Oliva-Teles A (2008) Temperature and dietary carbohydrate level effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 31:153–160. https://doi.org/10.1016/j.aquaculture.2007.11.016
Motta JHS, Ballester ELC, Souza AB, Polese MF, Radael MC, Gloria LS, Vidal MV (2023) Effect of periods of delayed first exogenous feeding in goldfish Carassius auratus (Linnaeus, 1758) larvae. Lat Am J Aquat Res 51(2):338–342
Mugwanya M, Dawood MAO, Kimera F, Sewilam H (2022) Anthropogenic temperature fluctuations and their effect on aquaculture: a comprehensive review. Aquac Fish 7:223–243. https://doi.org/10.1016/j.aaf.2021.12.005
Nadermann N, Seward RK, Volkoff H (2019) Effects of potential climate change -induced environmental modifications on food intake and the expression of appetite regulators in goldfish. Comp Biochem Physio 235:138–147
Ndong D, Chen YY, Lin YH, Vaseeharan B, Chen J (2007) The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shell Immun 22:686–694. https://doi.org/10.1016/j.fsi.2006.08.015
Ota KG, Abe G (2016) Goldfish morphology as a model for evolutionary developmental biology. Wiley Interdiscip Rev: Dev Biol 5(3):272–295
Palaksha KJ, Shin GW, Kim YR, Jung TS (2008) Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 24(4):479–488. https://doi.org/10.1016/j.fsi.2008.01.005
Pavlidis M, Karkana M, Fanouraki E, Papandroulakis N (2008) Environmental control of skin colour in the red porgy Pagrus Pagrus. Aquac Res 39(8):837–849. https://doi.org/10.1111/j.1365-2109.2008.01937.x
Peres H, Oliva-Teles A (1999) Influence of temperature on protein utilization in juvenile European seabass (Dicentrarchus labrax). Aquaculture 170(3):337–348. https://doi.org/10.1016/S0044-8486(98)00422-0
Pfeffer E, Beckmann-Toussaint J, Henrichfreise B, Jansen HD (1991) Effect of extrusion on efficiency of utilization of maize starch by rainbow trout (Oncorhynchus mykiss). Aquaculture 96(3):293–303. https://doi.org/10.1016/0044-8486(91)90159-5
Qi ZH, Liu YF, Luo SW, Chen CX, Liu Y, Wang WN (2013) Molecular cloning, characterization and expression analysis of tumor suppressor protein p53 from orange-spotted grouper, Epinephelus coioides in response to temperature stress. Fish Shellfish Immunol 35(5):1466–1476. https://doi.org/10.1016/j.fsi.2013.08.011
Roh H, Kim A, Kim N, Lee Y, Kim D (2020) Multi-omics analysis provides novel insight into immuno-physiological pathways and development of thermal resistance in rainbow trout exposed to acute thermal stress. Fish Immunol 21(23):9198. https://doi.org/10.3390/ijms21239198
Sarma K, Pal AK, Ayyappan S, Das T, Manush SM, Debnath D, Baruah K (2010) Acclimation of Anabas testudineus (Bloch) to three test temperatures influences thermal tolerance and oxygen consumption. Fish Physiol Biochem 36(1):85–90. https://doi.org/10.1007/s10695-008-9293-3
Scoffone E, Fontana A (1975) Proteins analysis. In: Needleman SB (ed) Protein sequence determination: a source book of methods and techniques. Springer-Verlag, New York, 162–203
Sun L, Chen H (2014) Effects of water temperature and fish size on growth and bioenergetics of cobia (Rachycentron canadum). Aquaculture 426:172–180. https://doi.org/10.1016/j.aquaculture.2014.02.001
Sun JL, Zhao LL, Wu H, Liu Q, Liao L, Luo J, Lian WQ, Cui C, Jin L, Ma JD, Li MZ, Yang S (2020) Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Sci Total Environ 713:135157. https://doi.org/10.1016/j.scitotenv.2019.135157
Svitacova K, Slavik O, Horky P (2023) Pigmentation potentially influences fish welfare in aquaculture. Appl Anim Behav Sci 262:105903. https://doi.org/10.1016/j.applanim.2023.105903
Talpur AD, Ikhwanuddin MH (2012) Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 364:6–12. https://doi.org/10.1016/j.aquaculture.2014.02.001
Tom L (1998) Nutritional and Feeding of Fish, 2nd edn. Kluwer Academic Publishers
Torrissen OJ, Naevdal G (1988) Pigmentation of salmonids - variation in flesh carotenoids of Atlantic salmon. Aquaculture 68(4):305–310. https://doi.org/10.1016/0044-8486(88)90244-X
Torrissen OJ, Hardy R, Shearer K (1989) Pigmentation of salmonids – carotenoid deposition and metabolism. Rev Aquat Sci 1:209–225
Torrissen OJ, Christiansen RR, Struksnæs G, Estermann R (1995) Astaxanthin deposition in the flesh of Atlantic Salmon, Salmo salar L., in relation to dietary astaxanthin concentration and feeding period. Aquac Nutr 1:77–84. https://doi.org/10.1111/j.1365-2095.1995.tb00022.x
Verma AK, Pal AK, Manush SM, Das T, Dalvi RS, Chandrachoodan PP, Ravi PM, Apte SK (2007) Persistent sub-lethal chlorine exposure augments temperature induced immunosuppression in Cyprinus carpio advanced fingerlings. Fish Shellfish Immunol 22(5):547–555. https://doi.org/10.1016/j.fsi.2006.08.001
Vissio PG, Darias MJ, Yorio MPD, Sirkin DIP, Delgadin TH (2020) Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. Gen Comp Endocrinol 301:113662. https://doi.org/10.1016/j.ygcen.2020.113662
Volkoff H, Ronnestad I (2020) Effects of temperature on feeding and digestive processes in fish. Temperature 7:307–320. https://doi.org/10.1080/23328940.2020.1765950
Wang N, Xu X, Kestemont P (2009) Effect of temperature and feeding frequency on growth performances, feed efficiency and body composition of pikeperch juveniles (Sander lucioperca). Aquaculture 289(1):70–73. https://doi.org/10.1016/j.aquaculture.2009.01.002
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Whangchai N, Chitmanat C, Ramarai R, Itayama T (2018) Effects of water flow rate and water quality on tilapia culture in the Mae ping river Thailand. Chiang Mai J Sci 45(3):1318–1322
Wiegertjes GF, Stet RJ, Parmentier HK, van Muiswinkel WB (1996) Immunogenetics of disease resistance in fish: a comparative approach. Dev Comp Immunol 20(6):365–381. https://doi.org/10.1016/s0145-305x(96)00032-8
Yanar M, Erdoğan E, Kumlu M (2019) Thermal tolerance of thirteen popular ornamental fish species. Aquaculture 501:382–386. https://doi.org/10.1016/j.aquaculture.2018.11.041
Zhao T, Ma A, Huang Z, Liu Z, Sun Z, Zhu C, Yang J, Li Y, Wang Q, Qiao X, Chen Z (2021) Transcriptome analysis reveals that high temperatures alter modes of lipid metabolism in juvenile turbot (Scophthalmus maximus) liver. Comp Biochem Physiol Part D Genomics Proteomics 40:100887. https://doi.org/10.1016/j.cbd.2021.100887
Zhou Q, Wang L, Wang H, Xie F, Wang T (2012) Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol 32(6):969–975. https://doi.org/10.1016/j.fsi.2012.01.024
Acknowledgements
All authors would like to thank the Department of Agriculture Science, Faculty of Agriculture Natural Resources and Environment, Naresuan University, Thailand, for the support and facilities used in this study.
Funding
This study was funded by the National Research Council of Thailand (NRCT, 65A107000053); the Fundamental Fund, Thailand: FF(NU)_2565 (grant number: FRB650022/0179, R2565B013).
Author information
Authors and Affiliations
Contributions
Anurak Khieokhajonkhet: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, supervision; Marisa Phoprakot: performed experiments, data collection; Niran Akesiri: hematological analysis; Gen Kaneko and Wutiporn Phromkunthong: review and editing the manuscript. The final manuscript was examined and accepted by all contributors.
Corresponding author
Ethics declarations
Ethics approval
All fish handling and experimental protocols were inspected and approved by The Faculty of Agriculture, Natural Resources and Environment, Naresuan University (Approval number: AG-AQ0002/2564). In addition, all experimental animal was also conducted in accordance with the guidelines of the Institute of Animals for Scientific Purpose Development (IAD), the National Research Council of Thailand's Ethic of Animal Experimentation (reference number: U1/00704/2558).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khieokhajonkhet, A., Phoprakot, M., Aeksiri, N. et al. Effects of thermal stress responses in goldfish (Carassius auratus): growth performance, total carotenoids and coloration, hematology, liver histology, and critical thermal maximum. Fish Physiol Biochem 49, 1391–1407 (2023). https://doi.org/10.1007/s10695-023-01263-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01263-9