Abstract
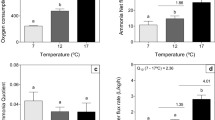
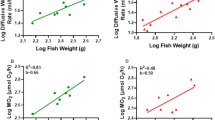
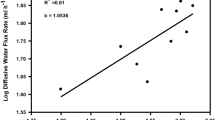
In nature, mosshead sculpins (Clinocottus globiceps) are challenged by fluctuations in temperature and oxygen levels in their environment. However, it is unclear how mosshead sculpins modulate the permeability of their branchial epithelia to water and O2 in response to temperature or hypoxia stress. Acute decrease in temperature from 13 to 6 oC reduced diffusive water flux rate by 22% and ṀO2 by 51%, whereas acute increase in temperature from 13 to 25 oC increased diffusive water flux rate by 217% and ṀO2 by 140%, yielding overall Q10 values of 2.08 and 2.47 respectively. Acute reductions in oxygen tension from >95% to 20% or 10% air saturation did not impact diffusive water flux rates, however, ṀO2 was reduced significantly by 36% and 65% respectively. During 1-h or 3-h recovery periods diffusive water flux rates were depressed while ṀO2 exhibited overshoots beyond the normoxic control level. Many responses differed from those seen in our parallel earlier study on the tidepool sculpin, a cottid with similar hypoxia tolerance but much smaller gill area that occupies a similar environment. Overall, our data suggest that during temperature stress, diffusive water flux rates and ṀO2 follow the traditional osmo-respiratory compromise pattern, but during hypoxia and re-oxygenation stress, diffusive water flux rates are decoupled from ṀO2.




Similar content being viewed by others
Data availability
Data and materials are available upon request
References
Blewett TA, Binning SA, Weinrauch AM, Ivy CM, Rossi GS, Borowiec BG, Lau GY, Overduin SL, Aragao I, Norin T (2022) Physiological and behavioural strategies of aquatic animals living in fluctuating environments. J Exp Biol 225:jeb242503. https://doi.org/10.1242/jeb.242503
Boutilier RG, Heming TA, Iwama GK (1984) Appendix: Physicochemical parameters for use in fish respiratory physiology. In: Hoar WS, Randall DJ (eds) Fish Physiology: Gills-Anatomy, Gas Transfer, and Acid-Base Regulation, vol 10. Part A. Academic Press, London, pp 404–430. https://doi.org/10.1016/S1546-5098(08)60323-4
Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K. et al. (2018) Declining oxygen in the global ocean and coastal waters. Science (80-.) 359, science.aam7240. https://doi.org/10.1126/science.aam7240
Cerda J, Finn RN (2010) Piscine aquaporins: an overview of recent advances. J Exp Zool 313A:623–650. https://doi.org/10.1002/jez.634
Cossins AR, Prosser CL (1978) Evolutionary adaptation of membranes to temperature. Proc Natl Acad Sc USA 75:2040–2043. https://doi.org/10.1073/pnas.75.4.2040
De Boeck G, Wood CM, Iftikar FI, Matey V, Scott GR, Sloman KA, Paula da Silva NM, Almeida-Val VMF, Val AL (2013) Interactions between hypoxia tolerance and food deprivation in Amazonian oscars, Astronotus ocellatus. J Exp Biol 216:4590–4600. https://doi.org/10.1242/jeb.082891
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(80):926–929. https://doi.org/10.1126/science.1156401
Evans DH (1969) Studies on the permeability to water of selected marine, freshwater and euryhaline teleosts. J Exp Biol 50:689–703 https://www.jstor.org/stable/30155750
Farrell AP, Richards JG (2009) Defining hypoxia: an integrative synthesis of the responses of fish to hypoxia. In Fish Physiology (Vol. 27, pp. 487-503). Academic Press
Giacomin M, Schulte PM, Wood CM (2017) Differential effects of temperature on oxygen consumption and branchial fluxes of urea, ammonia, and water in the dogfish shark (Squalus acanthias suckleyi). Physiol Biochem Zool 90:627–637. https://doi.org/10.1086/694296
Giacomin M, Onukwufor JO, Schulte PM, Wood CM (2020) Ionoregulatory aspects of the hypoxia-induced osmorespiratory compromise in the euryhaline killifish (Fundulus heteroclitus): the effects of salinity. J Exp Biol 223:jeb216309. https://doi.org/10.1242/jeb.216309
Gonzalez RJ, McDonald DG (1992) The relationship between oxygen consumption and ion loss in a freshwater fish. J Exp Biol 163:317–332. https://doi.org/10.1242/jeb.163.1.317
Gonzalez RJ, McDonald GD (1994) The relationship between oxygen uptake and ion loss in fish from diverse habitats. J Exp Biol 190:95–108
Hazel JR, Prosser CL (1974) Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev 54:620–677. https://doi.org/10.1152/physrev.1974.54.3.620
Hochachka P, Somero GN (2002) Biochemical adaptation: Mechanism and process in physiological evolution. Oxford University Press, Oxford
Holmes WN, Donaldson EM (1969) Body compartments and distribution of electrolytes. In: Hoar WS, Randall DJ (eds) Fish Physiology, vol 1. Academic Press, New York, pp 1–89. https://doi.org/10.1016/S1546-5098(08)60082-5
Iftikar FI, Matey V, Wood CM (2010) The ionoregulatory responses to hypoxia in the freshwater rainbow trout Oncorhynchus mykiss. Physiol Biochem Zool 83:343–355. https://doi.org/10.1086/648566
Isaia J (1984) Water and nonelectrolyte permeability. In: Hoar WS, Randall DJ (eds) Fish Physiology, vol 10B. Academic Press, San Diego, pp 1–38
Knope ML, Scales JA (2013) Adaptive morphological shifts to novel habitats in marine sculpin fishes. J Evol Biol 26:472–482. https://doi.org/10.1111/jeb.12088
Lopez-Patino MA, Hernandez-Perez J, Gesto M, Libran-Perez, Miguez JM, Soengas JL (2014) Short-term time course of liver metabolic response to acute handling stress in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol A Physiol 168:40–49
Loretz AC (1979) Water exchange across fish gills: the significance of tritiated-water flux measurements. J Exp Biol 79:147–162
Madsen SS, Engelund MB, Cutler CP (2015) Water transport and functional dynamics of aquaporins in osmoregulatory organs of fishes. Biol Bull 229:70–92. https://doi.org/10.1086/BBLv229n1p70
Mandic M, Sloman KA, Richards JG (2009a) Escaping to the surface: a phylogenetically independent analysis of hypoxia-induced respiratory behaviors in sculpins. Physiol Biochem Zool 82:730–738. https://doi.org/10.1086/605932
Mandic M, Todgham AE, Richards JG (2009b) Mechanisms and evolution of hypoxia tolerance in fish. Proc R Soc B Biol Sci 276:735–744. https://doi.org/10.1098/rspb.2008.1235
Mandic M, Speers-Roesch B, Richards JG (2013) Hypoxia tolerance in sculpins is associated with high anaerobic enzyme activity in brain but not in liver or muscle. Physiol Biochem Zool 86:92–105
Mandic M, Ramon ML, Gracey AY, Richards JG (2014) Divergent transcriptional patterns are related to differences in hypoxia tolerance between the intertidal and the subtidal sculpins. Mol Ecol 23:6091–6103. https://doi.org/10.1111/mec.12991
Matey V, Richards JG, Wang Y, Wood CM, Rogers J, Davies R, Murray BW, Chen XQ, Du J, Brauner CJ (2008) The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii. J Exp Biol 211:1063–1074
Matey V, Iftikar FI, De Boeck G, Scott GR, Sloman KA, Almeida-Val VMF, Val AL, Wood CM (2011) Gill morphology and acute hypoxia: responses of mitochondria-rich, pavement, and mucous cells in the Amazonian oscar (Astronotus ocellatus) and the rainbow trout (Oncorhynchus mykiss), two species with very different approaches to the osmo-respiratory compromise. Can J Zool 89:307–324. https://doi.org/10.1139/Z11-002
Nilsson S (1986) Control of gill blood flow. In: Nilsson S, Holmgren S (eds) Fish Physiology: Recent Advances. Croom Helm, London, pp 87–101. https://doi.org/10.1016/S1095-6433(97)00397-8
Olson KR (1992) Blood and extracellular fluid volume regulation. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish Physiology, vol 12B. Academic Press, San Diego, pp 135–254
Onukwufor JO, Wood CM (2018) The osmorespiratory compromise in rainbow trout (Oncorhynchus mykiss): The effects of fish size, hypoxia, temperature and strenuous exercise on gill diffusive water fluxes and sodium net loss rates. Comp Biochem Physiol A 219-220:10–18. https://doi.org/10.1016/j.cbpa.2018.02.002
Onukwufor JO, Wood CM (2020a) Reverse translation: effects of acclimation temperature and acute temperature challenges on oxygen consumption, diffusive water flux, net sodium loss rates, Q10 values and mass scaling coefficients in the rainbow trout (Oncorhynchus mykiss). J Comp Physiol B. https://doi.org/10.1007/s00360-020-01259-4
Onukwufor JO, Wood CM (2020b) Osmorespiratory compromise in zebrafish (Danio rerio): effects of hypoxia and thermal stress on oxygen consumption, diffusive water flux and sodium net loss rates. Zebrafish 17:400–411. https://doi.org/10.1089/zeb.2020.1947
Onukwufor JO, Wood CM (2022) The osmorespiratory compromise in marine flatfish: differential effects of temperature, salinity, and hypoxia on diffusive water flux and oxygen consumption of English sole (Parophrys vetulus) and Pacific sanddab (Citharichthys sordidus). Mar Biol 169:51. https://doi.org/10.1007/s00227-022-04040-z
Onukwufor JO, Kibenge F, Stevens D, Kamunde C (2016) Hypoxia-reoxygenation differentially alters the thermal sensitivity of complex I basal and maximal mitochondrial oxidative capacity. Comp Biochem Physiol A201:87–94. https://doi.org/10.1016/j.cbpa.2016.06.033
Onukwufor JO, Stevens D, Kamunde C (2017) Combined effect of cadmium, temperature and hypoxia-reoxygenation on mitochondrial function in rainbow trout (Oncorhynchus mykiss). Aquatic Toxicol 182:129–141. https://doi.org/10.1016/j.aquatox.2016.11.015
Pickering AD, Pottinger TG, Christie P (1982) Recovery of the brown trout, Salmo trutta L from acute handling stress: a time-course study. J Fish Biol 20:229–244
Pörtner HO (2012) Integrating climate-related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem-level changes. Mar Ecol Prog Ser 470:273–290. https://doi.org/10.3354/meps10123
Postlethwaite EK, McDonald DG (1995) Mechanisms of Na+ and Cl- regulation in freshwater-adapted rainbow trout (Oncorhynchus mykiss) during exercise and stress. J Exp Biol 198:295–304
Randall D (1982) The control of respiration and circulation in fish during exercise and hypoxia. J Exp Biol 100(1):275–288
Randall DJ, Daxboeck C (1984) Oxygen and carbon dioxide transfer across fish gills. In Richards J. G., Farrell A. P., & Brauner C. J. (Eds.), Fish Physiology (vol. 27, pp. 443–485). Academic Press
Randall DJ, Holeton GF, Stevens ED (1967) The exchange of oxygen and carbon dioxide across the gills of rainbow trout. J Exp Biol 46:339–348
Randall DJ, Baumgarten D, Malyusz M (1972) The relationship between gas and ion transfer across the gills of fishes. Comp Biochem Physiol 41A:629–637. https://doi.org/10.1016/0300-9629(72)90017-5
Richards JG (2009) Metabolic and molecular responses of fish to hypoxia. In Richards J. G., Farrell A. P., & Brauner C. J. (Eds.), Fish Physiology (vol. 27, pp. 443–485). Academic Press
Richards JG (2011) Metabolic rate suppression as a mechanism for surviving hypoxia. In: Farrell AP (ed) Encyclopedia of Fish Physiology: Energetics, Interactions with the Environment, Lifestyles, and Applications. Academic Press, Waltham, pp 1764–1770. https://doi.org/10.1007/978-3-642-02421-4_6
Robertson LM, Kochhann D, Bianchini A, Matey V, Almeida-Val VF, Val LA, Wood CM (2015a) Gill paracellular permeability and the osmorespiratory compromise during exercise in the hypoxia-tolerant Amazonian oscar (Astronotus ocellatus). J Comp Physiol B 185:741–754
Robertson LM, Val AL, Almeida-Val VF, Wood CM (2015b) Ionoregulatory aspects of the osmorespiratory compromise during acute environmental hypoxia in 12 tropical and temperate teleosts. Physiol Biochem Zool 88:357–370. https://doi.org/10.1086/681265
Ruhr IM, Wood CM, Schauer KL, Wang Y, Mager EM, Stanton B, Grosell M (2020) Is aquaporin-3 involved in water-permeability changes in the killifish during hypoxia and normoxic recovery, in freshwater or seawater? J Exp Zool 333:511–525. https://doi.org/10.1002/jez.2393
Saroglia M, Cecchini S, Terova G, Caputo A, De Stradis A (2000) Influence of environmental temperature and water oxygen concentration on gas diffusion distance in sea bass (Dicentrarchus labrax L.). Fish Physiol Biochem 23:55–58
Saroglia M, Terova G, De Stradis A, Caputo A (2002) Morphometric adaptations of sea bass gills to different dissolved oxygen partial pressures. J Fish Biol 60:1423–1430. https://doi.org/10.1111/j.1095-8649.2002.tb02437.x
Scott GR, Wood CM, Sloman KA, Iftikar FI, De Boeck G, Almeida-Val VMF, Val AL (2008) Respiratory responses to progressive hypoxia in the Amazonian oscar, Astronotus ocellatus. Resp Physiol Neurobiol 162:109–116. https://doi.org/10.1016/j.resp.2008.05.001
Sloman KA, Mandic M, Todgham AE, Fangue NA, Subrt P, Richards JG (2008) The response of the tidepool sculpin, Oligocottus maculosus, to hypoxia in laboratory, mesocosm and field environments. Comp Biochem Physiol A Mol Integr Physiol 149:284–292. https://doi.org/10.1016/j.cbpa.2008.01.004
Somo DA (2022) An integrative analysis of respiratory capacity, osmoregulatory function, body size, and metabolism in intertidal fishes (Doctoral dissertation, University of British Columbia)
Somo DA, Onukwufor JO, Wood CM, Richards JG (2020) Interactive effects of temperature and hypoxia on diffusive water flux and oxygen uptake rate in the tidepool sculpin, Oligocottus maculosus. Comp Biochem Physiol A 250:110781. https://doi.org/10.1016/j.cbpa.2020.110781
Somo DA, Chu K, Richards JG (2022) Aerobic scope falls to nil at Pcrit and anaerobic ATP production increases below Pcrit in the tidepool sculpin, Oligocottus maculosus. Biol Lett 18:20220342. https://doi.org/10.1098/rsbl.2022.0342
Somo DA, Chu K, Richards JG (2023) Gill surface area allometry does not constrain the body mass scaling of maximum oxygen uptake rate in the tidepool sculpin, Oligocottus maculosus. J Comp Physiol B. In revision.\
Sundin L, Nilsson GE (1997) Neurochemical mechanisms behind gill microcirculatory responses to hypoxia in trout: in vivo microscopy study. Am J Physiol 272:R576–R285. https://doi.org/10.1152/ajpregu.1997.272.2.R576
Tingaud-Sequeira A, Calusinska M, Finn RN, Chauvigné F, Lozano J, Cerdà J (2010) The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol Biol 10:1–18. https://doi.org/10.1186/1471-2148-10-38
Watters JV, Cech JJ Jr (2003) Behavioral responses of mosshead and woolly sculpins to increasing environmental hypoxia. Copeia 2003(2):397–401. https://doi.org/10.1643/0045-8511(2003)003[0397:BROMAW]2.0.CO;2
Wood CM, Eom J (2021) The osmorespiratory compromise in the fish gill. Comp Biochem Physiol A 254:110895. https://doi.org/10.1016/j.cbpa.2021.110895
Wood CM, Iftikar FI, Scott GR, De Boeck G, Sloman KA, Matey V, Valdez Domingos FX, Duarte RM, Almeida-Val VMF, Val AL (2009) Regulation of gill transcellular permeability and renal function during acute hypoxia in the Amazonian oscar (Astronotus ocellatus): new angles to the osmorespiratory compromise. J Exp Biol 212:1949–1964. https://doi.org/10.1242/jeb.028464
Wood CM, Ruhr IM, Schauer KL, Wang Y, Mager EM, McDonald D, Stanton B, Grosell M (2019) The osmorespiratory compromise in the euryhaline killifish: water regulation during hypoxia. J Exp Biol 222, pii: jeb204818. https://doi.org/10.1242/jeb.204818
Wu CB, Liu ZY, Li FG, Chen J, Jiang XY, Zou SM (2017) Gill remodeling in response to hypoxia and temperature occurs in the hypoxia sensitive blunt snout bream (Megalobrama amblycephala). Aquaculture 479:479–486
Funding
Supported by NSERC Discovery grants (RGPIN-2017-03843 to CMW and RGPIN-2020-04527 to JGR).
Author information
Authors and Affiliations
Contributions
The study was conceived by all authors. JOO and DAS performed all experiments and analyses. CMW and JGR obtained funding. JOO wrote the first draft of the manuscript, and all authors edited it.
Corresponding author
Ethics declarations
Ethical approval
Mosshead sculpins were collected under Fisheries and Oceans Canada scientific license XR-239-2017. All experiments were done under the approved animal protocols of both Bamfield Marine Sciences Centre (BMSC) (BSMC AUP RS-17-11) and the University of British Columbia (UBC AUP A13-0309) in accordance with the Canadian Council on Animal Care guidelines
Competing interests
All authors declare no competing interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Onukwufor, J.O., Somo, D.A., Richards, J.G. et al. Osmo-respiratory compromise in the mosshead sculpin (Clinocottus globiceps): effects of temperature, hypoxia, and re-oxygenation on rates of diffusive water flux and oxygen uptake. Fish Physiol Biochem 49, 853–866 (2023). https://doi.org/10.1007/s10695-023-01226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01226-0