Abstract
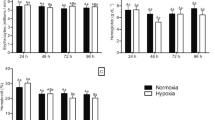
Hypoxia has gradually become common in aquatic ecosystems and imposes a significant challenge for fish farming. The loss of equilibrium (LOE), 50% lethal time (LT50), plasma cortisol, glucose, red blood cells (RBC), hemoglobin (Hb), gill histological alteration, and related parameters (lamellar length [SLL] and width [SLW], interlamellar distance [ID], basal epithelial thickness [BET], lamellar surface area [LA], and gill surface area [GSA]); respiratory rate; the proportion of the secondary lamellae available for gas exchange (PAGE); and hypoxia-inducible factor (hif-1α, hif-2α) mRNA expression were determined during progressive hypoxia and reoxygenation (R-0, R-12, R-24 h) to illustrate the underlying physiological response mechanisms in black rockfish Sebastes schlegelii. Results showed that the DO concentration significantly decreased during progressive hypoxia, while DO at LOE and LT50 were 2.42 ± 0.10 mg L−1 and 1.67 ± 0.38 mg L−1, respectively. Cortisol and glucose were significantly increased at LOE and LT50, with the highest levels observed at LT50, and then gradually recovered to normal within reoxygenation 24 h. RBC number and Hb results were like those of glucose. Hypoxia stress resulted in lamellar clubbing, hypertrophy, and hyperplasia. Respiratory frequency significantly increased at LOE and decreased at LT50. Lamellar perimeters, SLL, ID, LA, GSA, and PAGE, significantly increased at LOE and LT50, with the highest values observed at LT50. However, SLW and BET significantly decreased at LOE, LT50, and R-0. These parameters recovered to nearly normal levels at R-24 h. hif-1α mRNAs in gill and liver were significantly upregulated at LOE and LT50, and recovery to normal after reoxygenation 24 h. hif-2α mRNAs in gill was similar to that of hif-1α, whereas hepatic hif-2α mRNAs remained unchanged during hypoxia-reoxygenation. These results indicated that progressive hypoxia stress elevated RBC number, Hb, cortisol, and glucose levels, induced the alteration of gill morphology, increased LA and GSA, stimulated respiratory frequency and PAGE, and upregulated the transcription of hif-1α and hif-2α in gill and liver. Reoxygenation treatment for 24 h alleviated the stress mentioned above effects. These findings expand current knowledge on hypoxia tolerance in black rockfish Sebastes schlegelii.








Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Abdel-Tawwab M, Hagras AE, Elbaghdady HM, Monier MN (2014) Dissolved oxygen level and stocking density effects on growth, feed utilization, physiology, and innate immunity of Nile tilapia, Oreochromis niloticus. J Appl Aquac 26:340–355
Abdel-Tawwab M, Monier MN, Hoseinifar SH, Faggio C (2019) Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol Biochem 45:997–1013
Affonso EG, Polez VL, Correa CF, Mazon AF, Araujo MR, Moraes G, Rantin FT (2002) Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comp Biochem Physiol C 133:375–382
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115
Al-Ghanbousi R, Ba-Omar T, Victor R (2012) Effect of deltamethrin on the gills of Aphanius dispar: a microscopic study. Tiss Cell 44:7–14
Baker BI, Rance TA (1981) Differences in concentrations of plasma cortisol in the trout and the eel following adaptation to black or white backgrounds. J Endocrionol 89:135–140
Benli ACK, Köksal G, Özkul A (2008) Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): effects on gill liver and kidney histology. Chemosphere 72:1355–1358
Blanco AM (2020) Hypothalamic- and pituitary-derived growth and reproductive hormones and the control of energy balance in fish. Gen Comp Endocrinol 287:113322
Boran H, Altinok I, Capkin E (2010) Histopathological changes induced by maneb and carbaryl on some tissues of rainbow trout, Oncorhynchus mykiss. Tiss Cell 42:158–164
Bowden AJ, Gardiner NM, Couturier CS, Stecyk JA, Nilsson GE, Munday PL, Rummer JL (2014) Alterations in gill structure in tropical reef fishes as a result of elevated temperatures. Comp Biochem Physiol A 175:64–71
Brauner CJ, Rombough PJ (2012) Ontogeny and paleo physiology of the gill: new insights from larval and air-breathing fish. Respir Physiol Neurobiol 184:293–300
Brett JR (1979) Environmental factors and growth. In: Fish physiology, vol. VIII. In: Hoar WS, Randall DJ, Brett JR (eds) Academic Press, New York, pp 599–675
Capaldo A, Gay F, Laforgia V (2019) Changes in the gills of the European eel (Anguilla anguilla) after chronic exposure to environmental cocaine concentration. Ecotoxicol Environ Saf 169:112–119
Chen N, Chen LP, Zhang J, Chen C, Wei XL, Gul Y, Wang WM, Wang HL (2012) Molecular characterization and expression analysis of three hypoxia inducible factor alpha subunits, HIF-1α/2α/3α of the hypoxia-sensitive freshwater species, Chinese sucker. Gene 498:81–90
Choi E, Alsop D, Wilson JY (2018) The effects of chronic acetaminophen exposure on the kidney, gill and liver in rainbow trout (Oncorhynchus mykiss). Aqua Toxicol 198:20–29
Choudhry H, Harris AL (2018) Advances in Hypoxia-inducible factor biology. Cell Metab 27(2):281–298
Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through Fry’s concept of aerobic metabolic scope. J Fish Biol 88:232–251
Cook DG, Herbert NA (2012) The physiological and behavioural response of juvenile kingfish (Seriola lalandi) differs between escapable and inescapable progressive hypoxia. J Exp Mar Biol Ecol 413:138–144
Crespo S, Sala R, Marlasca MJ (1987) Different cell types in the gill epithelium of juvenile turbot (Scophthalmus maximus). Aquaculture 67:216–217
Dacie JV, Lewis SM (1991) Practical haematology, 7th edn. Churchill Livingstone, London
Dejours P (1975) Principles of comparative respiratory physiology. North Holland, Amsterdam, pp 1-253
Dhillon RS, Yao L, Matey V, Chen BJ, Zhang AJ, Cao ZD, Fu SJ, Brauner CJ, Wang YS, Richards JG (2013) Interspecifc differences in hypoxia- induced gill remodeling in carp. Physiol Biochem Zool 86:727–739
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Douxfils J, Deprez M, Mandiki SN, Milla S, Henrotte E, Mathieu C et al (2012) Physiological and proteomic responses to single and repeated hypoxia in juvenile Eurasian perch under domestication-clues to physiological acclimation and humoral immune modulations. Fish Shellfish Immunol 33:1112–1122
Faraway JJ (2005) Linear models with R. Chapman & Hall/CRC, Boca Raton
Gandar A, Laffaille P, Canlet C, Tremblay-Franco M, Gautier R, Perrault A et al (2017) Adaptive response under multiple stress exposure in fish: from the molecular to individual level. Chemosphere 188:60–72
Gilmore KL, Doubleday ZA, Gillanders BM (2019) Prolonged exposure to low oxygen improves hypoxia tolerance in a freshwater fish. Conser Physiol 1:58
Gisbert E, Rodriguez A, Cardona L, Huertas M, Gallardo MA, Sarasquete C, SalaRabanal M, Ibarz A, Sánchez J, Castelló-Orvay F (2004) Recovery of Siberian sturgeon yearlings after an acute exposure to environmental nitrite: changes in the plasmatic ionic balance, Na+-K+ ATPase activity and gill histology. Aquaculture 239:141–154
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC (2003) Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol 23:9361–9374
Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC (2006) Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 Alpha (HIF-1α) and HIF-2α in stem cells. Mol Cell Biol 26:3514–3526
Jee BY, Do YH, Min BH, Park MS, Hwang HG, Myeong JI, Cho JK (2015) Korean J Environ Biol 33(4):412–418
Jenny JP, Francus P, Normandeau A, Lapointe F, Perga ME, Ojala A, Schimmelmann A, Zolitschka B (2016) Global spread of hypoxia in freshwater ecosystems during the last three centuries is caused by rising local human pressure. Glob Chang Biol 22:1481–1489
Johansson D, Laursen F, Fernӧ A, Fosseidengen JE, Klebert P, Stien LH, Vågseth T, Oppedal F (2014) The interaction between water currents and salmon swimming behaviour in sea cages. PLoS One 9:e97635
Jung JH, Kim HN, Chae YS, Shim WJ (2014) Biochemical responses of Juvenile rockfish (Sebastes schlegeli) to low levels of dissolved oxygen in Gamak Bay. Ocean Sci J 49(3):241–247
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–1480
Kim KH, Hwang YJ, Kwon SR (2001) Influence of daily water temperature changes on the chemiluminescent response and mortality of cultured rockfish (Sebastes schlegeli). Aquaculture 192:93–99
Kramer DL, McClure M (1982) Aquatic surface respiration, a widespread adaptation to hypoxia in tropical freshwater fishes. Environ Biol Fish 7:47–55
Law SHW, Wu RSS, Ng PKS, Yu RMK, Kong RYC (2006) Cloning and expression analysis of two distinct HIF-alpha isoforms-gcHIF-1alpha and gcHIF-4alpha from the hypoxia-tolerant grass carp ( Ctenopharyngodon idellus). BMC Mol Biol 7:1–15
Lawrence MJ, Eliason EJ, Zolderdo AJ, Lapointe D, Best C, Gilmour KM, Cooke SJ (2019) Cortisol modulates metabolism and energy mobilization in wild-caught pumpkinseed (Lepomis gibbosus). Fish Physiol Biochem 45:1813–1828
Lays N, Iversen MMT, Frantzen M, Jorgensen EH (2009) Physiological stress responses in spotted wolfish (Anarhichas minor) subjected to acute disturbance and progressive hypoxia. Aquaculture 295:126–133
Liebel S, Tomotake MEM, Ribeiro CAO (2013) Fish histopathology as biomarker to evaluate water quality. Ecotoxicol Environ Contam 8(2):9–15
Lin HR, Liu XC (2006) The experimental methods of fish physiology[M] pp: 27–29 Guanzhou Guangdong: Guanzhou High Education Press
Liu Y, Ma D, Zhao C, Xiao Z, Xu S, Xiao Y, Wang Y, Liu Q, Li J (2017) The expression pattern of hsp70 plays a critical role in thermal tolerance of marine demersal fish: Multilevel responses of Paralichthys olivaceus and its hybrids (P. olivaceus ♀×P. dentatus ♂) to chronic and acute heat stress. Mar Environ Res 129:386–395
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Love MS, Yoklavich M, Thorsteinson LK (2002) The rockfishes of the northeast Pacific: University of California Press
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can j Fish Aquat Sci 42:630–648
Mitrovic D, Agnieszka D, Nilsson GE, Perry SF (2009) Physiological consequences of gill remodeling in goldfish (Carassius auratus) during exposure to long-term hypoxia. Am J Physiol Regul Integr Comp Physiol 297:224–234
Mohindra V, Tripathi RK, Singh RK, Lal KK (2013) Molecular characterization and expression analysis of three hypoxia-inducible factor alpha subunits, HIF-1α, -2α and -3α in hypoxia-tolerant Indian catfish (Clarias batrachus ) [Linnaeus, 1758]. Mol Biol Rep 40:5805–5815
Moraes G, Avilez IM, Altran AE, Barbosa CC (2002) Biochemical and hematological responses of the banded knife fish Gymnotus carapo (Linnaeus, 1758) exposed to environmental hypoxia. Braz J Biol 62:633–640
Mu W, Wen H, Li J, He F (2015) HIFs genes expression and hematology indices responses to different oxygen treatments in an ovoviviparous teleost species Sebastes schlegelii. Mar Env Res 110:142–151
Nero V, Farwell A, Lister A, Van der Kraak G, Lee LE, Van Meer T, MacKinnon MD, Dixon DG (2006) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Safe 63(3):365–377
Nilsson GE (2007) Gill remodeling in fish-a new fashion or an ancient secret? J Exp Biol 210:2403–2409
Nilsson GE, Dymowska A, Stecyk JA (2012) New insights into the plasticity of gill structure. Respir Physiol Neurobiol 184:214–222
Oppedal F, Vågseth T, Dempster T, Juell JE, Johansson D (2011) Fluctuating sea cage environments modify the effects of stocking densities on production and welfare parameters of Atlantic salmon (Salmo salar L.). Aquaculture 315:361–368
Pan YK, Perry SF (2020) Neuroendocrine control of breathing in fish. Mol Cell Endocrinol 509:110800
Perry SF, Fletcher C, Bailey S, Ting J, Bradshaw J, Tzaneva V, Gilmour KM (2012) The interactive effects of exercise and gill remodeling in goldfish (Carassius auratus). J Comp Physiol B 182:935–945
Pichavant K, Maxime V, Thébault MT, Ollivier H, Garnier JP, Bousquet B, Diouris M, Boeuf G, Nonnotte G (2002) Effects of hypoxia and subsequent recovery on turbot Scophtalmus maximus: hormonal changes and anaerobic metabolism. Mar Ecol Prog Ser 225:275–285
Powell WH, Hahn ME (2002) Identification and functional characterization of hypoxia-inducible factor 2a from the estuarine teleost, Fundulus heteroclitus: interaction of HIF-2α with two ARNT2 splice variants. J Exp Zool A Ecol Genet Physiol 294:17–29
Rahman MS, Thomas P (2007) Molecular cloning, characterization and expression of two hypoxia-inducible factor alpha subunits, HIF-1α and HIF-2α, in a hypoxia-tolerant marine teleost, Atlantic croaker (Micropogonias undulatus). Gene 396:273–282
Reiser S, Wuertz S, Schroeder JP, Kloas W, Hanel R (2011) Risks of seawater ozonation in recirculation aquaculture – effects of oxidative stress on animal welfare of juvenile turbot (Psetta maxima, L.). Aquat Toxicol 105:508–517
Rimoldi S, Terova G, Ceccuzzi P, Marelli S, Antonini M, Saroglia M (2012) HIF-1a mRNA levels in Eurasian perch (Perca fluviatilis) exposed to acute and chronic hypoxia. Mol Biol Rep 39:4009–4015
Rodrigues S, Antunes SC, Nunes B, Correia AT (2017) Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environ Toxicol Pharmacol 53:164–176
Rombough P (2007) The functional ontogeny of the teleost gill: which comes first, gas or ion exchange? Comp Biochem Physiol A 148:732–742
Rotllant J, Guerreiro PM, Anjos L, Redruello B, Canario AVM, Power DM (2005) Stimulation of cortisol release by the N terminus of teleost parathyroid hormone-related protein in interrenal cells in vitro. Endocrinology 146:71–76
Semenza GL (1998) Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 8:588–594
Semenza GL (2000) HIF-1: mediator of physiological and pathophysiologic al responses to hypoxia. J Appl Physiol 88:1474–1480
Semenza GL (2001) HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3
Sollid J, Nilsson GE (2006) Plasticity of respiratory structures-adaptive remodelling of fish gills induced by ambient oxygen and temperature. Respir Physiol Neurobiol 154:241–251
Sollid J, Angelis PD, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. Exp Biol 206:3667–3673
Sollid J, Kjernsli A, De Angelis PM, Rohr AK, Nilsson GE (2005) Cell proliferation and gill morphology in anoxic crucian carp. Am J Phys Regul Integr Comp Phys 289:1196–1201
Solstorm D, Oldham T, Solstorm F, Klebert P, Stien LH, Vågseth T, Oppedal F (2018) Dissolved oxygen variability in a commercial sea-cage exposes farmed Atlantic salmon to growth limiting conditions. Aquaculture 486:122–129
Stierhoff KL, Targett TE, Grecay PA (2003) Hypoxia tolerance of the mummichog: the role of access to the water surface. J Fish Biol 63:580–592
Sula E, Aliko V (2017) Effects of stressors on hematological and immunological response in fresh water crucian carp fish (Carassius carassius). Albanian J Agric Sci 1:583–950
Sun JL, Zhao LL, Wu H, Liu Q, Liao L, Luo J et al (2020) Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Sci Total Env 713:135157
Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, Manabe I, Nagai R (2004) Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res 95:146–153
Terova G, Rimoldi S, Cora S, Bernardini G, Gornati R, Saroglia M (2008) Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax). Aquaculture 279:150–159
Thomas LW, Mcnulty ST, Klesius PH (2007) Effect of sublethal hypoxia on the immune response and susceptibility of channel catfish, Ictalurus punctatus to enteric septicemia. J World Aquacult Soc 38:12–23
Tzaneva V, Bailey S, Perry SF (2011) The interactive effects of hypoxemia, hyperoxia, and temperature on the gill morphology of goldfish (Carassius auratus). Am J Physiol Regul Integr Comp Physiol 300:1344–1351
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. PNAS 105(40):15452–15457
Wang QF, Shen WL, Hou CC, Liu C, Wu XF, Zhu JQ (2017) Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia. Chemosphere 169:418–427
Weber JM, Choi K, Gonzalez A, Teye O (2016) Metabolic fuel kinetics in fish: swimming, hypoxia and muscle membranes. J Exp Biol 219:250–258
Wells RMG, Baldwin J (2006) Plasma lactate and glucose flushes following burst swimming in silver trevally (Pseudocaranx dentex: Carangidae) support the “releaser” hypothesis. Comp Biochem Physiol A 143:347–352
Wenger RH, Gassmann M (1997) Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem 378:609–616
Wood AT, Clark TD, Elliott NG, Frappell PB, Andrewartha SJ (2020) The effects of constant and cyclical hypoxia on the survival, growth and metabolic physiology of incubating Atlantic salmon (Salmo salar). Aquaculture 527:735449
Wu ZH, You F, Wen AY, Ma DY, Zhang PJ (2016) Physiological and morphological effects of severe hypoxia, hypoxia and hyperoxia in juvenile turbot (Scophthalmus maximus L.). Aquac Res 47:219–227
Wu CB, Liu ZY, Li FG, Chen J, Jiang XY, Zou SM (2017) Gill remodeling in response to hypoxia and temperature occurs in the hypoxia sensitive blunt snout bream (Megalobrama amblycephala). Aquaculture 479:479–486
Wu CB, Zheng GD, Zhao XY, Zhou S, Zou SM (2020) Hypoxia tolerance in a selectively bred F4 population of blunt snout bream (Megalobrama amblycephala) under hypoxic stress. Aquaculture 518:734484
Yang H, Cao ZD, Fu SJ (2013) The effects of diel-cycling hypoxia acclimation on the hypoxia tolerance, swimming capacity and growth performance of southern catfish (Silurus meridionalis). Comp Biochem Physiol A 165:131–138
Zhao L, Cui C, Liu Q, Sun J, He K, Adam AA (2020) Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquatic Toxicol 224:105514
Funding
This study was supported by National Key R&D Program of China (2019YFD0900904), Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022020008, 2020TD51), and Key Project of Shandong Provincial Natural Science foundation (ZR202010310016).
Author information
Authors and Affiliations
Contributions
Yudong Jia mainly designed experiment, wrote, and revised the manuscript. Yuntao Gao finished hematological and biochemical parameter analysis, and gill histology experiment. Jinming Wan and Yunhong Gao are responsible for qRT-PCR experiment. Juan Li checked the grammar, word usage, and give more helpful comments. Changtao Guan provided partly funding support. All authors joined the analysis and interpretation of data and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were conducted according to the guidelines established by the Institutional Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Science.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jia, Y., Gao, Y., Wan, J. et al. Altered physiological response and gill histology in black rockfish, Sebastes schlegelii, during progressive hypoxia and reoxygenation. Fish Physiol Biochem 47, 1133–1147 (2021). https://doi.org/10.1007/s10695-021-00970-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-00970-5