Abstract
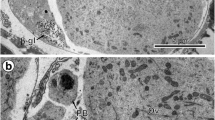
Egg-envelope, an acellular coat, surrounds the egg and is essential for vitellogenin incorporation. It also plays a pivotal role during fertilization and provides protection to the developing embryo. In the present study, scanning electron microscopy was used to elucidate the structural details of isolated egg-envelopes from the Indian freshwater murrel, Channa punctatus. Several pores and single micropyle were observed on outer surface, whereas inner layer indicated deposition of proteinaceous material. The constituent proteins of egg-envelope were further characterized by Fourier transform infrared (FT-IR) spectroscopy, and electrophoresis and mass-spectrometry (MALDI-TOF-MS/MS). The secondary structure of egg-envelope proteins showed the presence of antiparallel ß-pleated sheets and aromatic amino acids. These proteins resolved into two peptides (130 kDa and 68 kDa) under denaturing conditions, which exhibited glycoprotein nature. The peptide band with low molecular mass showed significant similarity with transmembrane protein, whereas peptide band with high molecular mass matched with choriogenin protein of other fishes. These results confirm that chorion is derived from precursor protein, Choriogenin, in murrel. Chemical composition of egg-envelope supports that chorion is responsible exchange material and chemical defence during embryogenesis.






Similar content being viewed by others
References
Arukwe A, Goksoyr A (2003) Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp Hepatol 2:4
Baldacci A, Taddei A, Mazzini M, Fausto A, Buonocore F, Scapigliati G (2001) Ultrastructure and proteins of the egg chorion of the antarctic fish Chionodraco hamatus (Teleostei, Notothenioidei). Polar Biol 24:417–421
Barth A (2000) The infrared absorption of amino acid side chains. Prog Biophys Mol Biol 74:141–173
Berois N, Arezo MJ, Papa NG (2011) Gamete interactions in teleost fish: the egg envelope. Basic studies and perspectives as environmental biomonitor. Biol Res 44:119–124
Bless R, Riehl R (2002) Biology and egg morphology of the Dalmatian barbelgudgeon Aulopyge huegeli, an endangered endemic species in Croatia. Environ Biol Fish 63:451–456
Chen CH, Wu CC, Shao KT, Yang JS (2007) Chorion microstructure for identifying five fish eggs of Apogonidae. J Fish Biol 71:913–919
Clark AE, Kaleta EJ, Arora A, Wolk DM (2013) Matrix-assisted laser desorption ionization–time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev 26:547–603
Coward K, Bromage NR, Hibbitt O, Parrington J (2002) Gamete physiology, fertilization and egg activation in teleost fish. Rev Fish Biol Fish 12:33–58
Cutting JA, Roth TF (1973) Staining of phospho-proteins on acrylamide gel electropherograms. Anal Biochem 54:386–394
Darie CC, Biniossek ML, Jovine L, Litscher ES, Wassarman P (2004) Structural characterization of fish egg vitelline envelope proteins by mass spectrometry. Biochemistry 43:7459–7478
Domon B, Aebersold R (2006) Mass spectrometry and protein analysis. Science 312:212–217
Esmaeili HR, Gholamifard A (2012) Ultrastructure of the chorion and the micropyle of an endemic cyprinid fish, Cyprinion tenuiradius Heckel, 1849 (Teleostei: Cyprinidae) from southern Iran. Iran J Fish Sci 11:657–665
Esmaeili HR, Johal MS (2005) Ultrastructural features of the egg envelope of silver carp, Hypophthalmichthys molitrix (Osteichthyes, Cyprinidae). Environ Biol Fish 72:373–377
Fujiwara Y, Fukada H, Shimizu M, Hara A (2005) Purification of two lipovitellins and development of immunoassays for two forms of their precursors (vitellogenins) in medaka (Oryzias latipes). Gen Comp Endocrinol 143:267–277
Gibbs AG, Rajpurohit S (2009) Cuticular lipids and water balance. In: Blomquist GJ, Bagne’res AG (eds) Insect hydrocarbons. Cambridge University Press, Cambridge, pp 100–120
Glechner R, Patzner RA, Riehl R (1993) Die Eier heimischer Fische. 5. Schneider – Alburnoides bipunctata (Bloch, 1782) (Cyprinidae). Österreichs Fischerei 46:169–172
Griebenow K, Santos AM, Carrasquillo KG (1999) Secondary structure of proteins in the amorphous dehydrated state probed by FTIR spectroscopy. Dehydration-induced structural changes and their prevention. Internet J Vibr Spectr 3(1):1–34
Hara A, Hiramatsu N, Fujita T (2016) Vitellogenesis and choriogenesis in fishes. Fish Sci 82(2):187–202
Hoodbhoy T, Dean J (2004) Insights into the molecular basis of sperm–egg recognition in mammals. Reproduction 127:417–422
Iconomidou VA, Chryssikos DG, Gionis V, Pavlidis MA, Paipetis A, Hamodrakas SJ (2000) Secondary structure of chorion proteins of the teleostean fish Dentex dentex by ATR FT-IR and FT-Raman spectroscopy. J Struct Biol 132:112–122
Izumi H, Hagihara S, Kurogi H, Chow S, Tsukamoto K, Kagawa H, Adachi S (2015) Histological characteristics of the oocyte chorion in wild post-spawning and artificially matured Japanese eels Anguilla japonica. Fish Sci 81:321–329
Kapitany RA, Zebrowski EJ (1973) A high-resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem 56:361–369
Killingbeck EE, Wilburn DB, Merrihew GE, MacCoss MJ, Swanson W (2020) Proteomics support the three-spine stickleback egg coat as a protective oocyte envelope. bioRxiv
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lahnsteiner F (2003) The spermatozoan and eggs of the cardinal fish. J Fish Biol 62:115–128
Lee C, Na JG, Lee KC, Park K (2002) Choriogenin mRNA induction in male medaka, Oryzias latipes as a biomarker of endocrine disruption. Aquat Toxicol 61(3–4):233–241
Li YH, Wu CC, Yang JS (2000) Comparative ultrastructural studies of the zona radiata of marine fish eggs in three genera in Perciformes. J Fish Biol 56:615–621
Litscher ES, Wassarman PM (2018) The fish Egg's zona Pellucida. Curr Top Dev Biol 130:275–305
Lubzens E, Bobe J, Young G, Sullivan CV (2017) Maternal investment in fish oocytes and eggs: the molecular cargo and its contributions to fertility and early development. Aquacult 472:107–143
Messaddeq N, Hergueux J, Weickert JL, Romand R (2018) Study of the chorion of seasonal and non-seasonal Africa and Neotropical oviparous Cyprinodontiforme fishes. Environ Biol Fish 101(2):287–299
Modig C, Westerlund L, Olsson PE (2007) Oocyte zona pellucida proteins. In: Babin PJ, Cerda J, Lubzens E (eds) The fish oocyte: from basic studies to biotechnological applications. Springer, Dordrecht, pp 113–139
Murata K, Conte FS, McInnis E, Fong TH, Cherr GN (2014) Identification of the origin and localization of chorion (egg envelope) proteins in an ancient fish, the white sturgeon, Acipenser transmontanus. Biol Reprod 90:132–131
Orfanidou CC, Hamodrakas SJ, Chryssikos GD, Kamitsos EI, Wellman SE, Case ST (1995) Spectroscopic studies of Manduca sexta and Sesamia nonagrioides chorion protein structure. Int J Biol Macromol 17(2):93–98
Palumbo AJ, Linares-Casenave J, Jewell W, Doroshov SI, Tjeerdema RS (2007) Induction and partial characterization of California halibut (Paralichthys californicus) vitellogenin. Comp Biochem Physiol A Mol Integr Physiol 146:200–207
Patzner RA, Riehl R, Glechner R (1996) Die Eier heimischer Fische. 11. Plötze – Rutilus rutilus (Linnaeus, 1758) und Perlfisch – Rutilus frisii meidingeri (Heckel, 1852) (Cyprinidae). Fischökologie 9:15–26
Prakash O, Goswami SV, Sehgal N (2007) Establishment of ELISA for murrel vitellogenin and choriogenin, as biomarkers of potential endocrine disruption. Comp Biochem Physiol C Toxicol Pharmacol 146:540–551
Rani KV, Sehgal N, Goswami SV, Prakash O (2010) Relative potencies of natural estrogens on vitellogenin and choriogenin levels in the Indian freshwater spotted snakehead, Channa punctatus: in vivo and in vitro studies. Fish Physiol Biochem 36(3):587–595
Riehl R (1993) Surface morphology and micropyle as a tool for identifying fish eggs by scanning electron microscopy. Eur Microsc Anal 5:29–31
Riehl R, Patzner RA (1994) Die Eier heimischer Fische. 7. Karpfen – Cyprinus carpio Linnaeus, 1758 (Cyprinidae). Acta Biol Benrodis 6:1–7
Riehl R, Patzner RA, Glechner R (1993) Die Eier heimischer Fische. 2. Seelaube – Chalcalburnus chalcoides mento (Agassiz, 1832). Österreichs Fischerei 46:138–140
Riehl R, Arnold A, Patzner RA (1995) Die Eier heimischer Fische. 10. Moderlieschen – Leucaspius delineatus (Heckel, 1843) (Cyprinidae). Z Fischkunde 3:77–89
Sano K, Kawaguchi M, Katano K, Tomita K, Inokuchi M, Nagasawa T, Arai K (2017) Comparison of egg envelope thickness in teleosts and its relationship to the sites of ZP protein synthesis. J Exp Zool B Mol Dev Evol 328:240–258
Sehgal N, Goswami SV (2002) Immunological identification of two female-specific proteins from the plasma of Indian freshwater murrel, Channa punctatus. Indian J Exp Biol 40:288–295
Srivastava AK, Iconomidou VA, Chryssikos GD, Gionis V, Kumar K, Hamodrakas SJ (2011) Secondary structure of chorion proteins of the Lepidoptera Pericallia ricini and Ariadne merione by ATR FT-IR and micro-Raman spectroscopy. Int J Biol Macromol 49:317–322
Venyaminov SY, Kalnin NN (1990) Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. II. Amide absorption bands of polypeptides and fibrous proteins in 훼-, 훽-, and random coil conformations. Biopolymers 30:1259–1271
Verdú JR, Gallego B, Lobo JM, Ortiz AJ, Cortez V, Halffter G (2014) Intra-population variation and geographic correlation in Canthon humectus hidalgoensis using FTIR-ATR spectroscopy. Ecol Res 29:1105–1113
Wassarman PM (2008) Zona pellucida glycoproteins. J Biol Chem 283:24285–24289
Yanagimachi R, Harumi T, Matsubara H, Yan W, Yuan S, Hirohashi N, Andoh T (2017) Chemical and physical guidance of fish spermatozoa into the egg through the micropyle. Biol Reprod 96(4):780–799
Yue HM, Cao H, Chen XH, Ye H, Li CJ, Du H (2014) Molecular characterization of the cDNA s of two zona pellucida genes in the Chinese sturgeon, Acipenser sinensis (Gray, 1835). J Appl Ichthyol 30(6):1273–1281
Funding
The present work was supported by Research Grant from the University of Delhi, Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vijay, P., Sehgal, N. Structural analysis and characterization of egg-envelope in the Indian freshwater murrel, Channa punctatus. Fish Physiol Biochem 46, 1847–1856 (2020). https://doi.org/10.1007/s10695-020-00834-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00834-4