Abstract
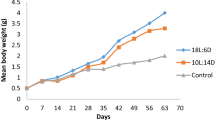
Photoperiod is important in initiation or suppression of reproductive timing and gonadal maturation which varies with species. The aim of the present study was to investigate the effect of two photoperiodic manipulating regimes, i.e., long (18L:6D) and short (10L:14D) photoperiods for a period of 60 days on somatic growth and gonadal maturation of a live-bearer ornamental fish, Mickey Mouse platy (Xiphophorus maculatus). The control fish were further kept under the laboratory environmental condition. The results showed a significant increase in weight gain, specific growth rate, and gonadosomatic index in fish under long photoperiod than those exposed to short photoperiod and control condition (P < 0.05). A condition factor showed significant variations between long photoperiod and control groups. Furthermore, a long photoperiod also induced a significant increase in the number of fish with mature embryo and middle-eyed embryo in the ovary. Similarly, histological analysis of testes of males showed an increase in the number of mature spermatid and spermatozoa under long photoperiod when compared to those of control and short photoperiod ones. Thus, it can be concluded that long-day photoperiodic manipulation may be applied for healthy growth and early gonadal maturation of live-bearer ornamental fishes.







Similar content being viewed by others
References
Alamazan Rueda P, Helmond ATM, Verreth JAJ, Schrama JW (2005) Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus. J Fish Biol 67:1029–1039
APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater (21st Ed). American Public Health Association, Washington, DC
Ballagh DA, Pankhurst PM, Fielder DS (2008) Photoperiod and feeding interval requirements of juvenile mulloway, Argyrosomus Japonicus. Aquaculture 277:52–57
Bapary MAJ, Takemura A (2010) Effect of temperature and photoperiod on the reproductive condition and performance of a tropical damselfish Chrysiptera cyanea during different phases of the reproductive season. Fish Sci 76:769–776
Barimani S, Kazemi MB, Hazaei K (2013) Effects of different photoperiod regimes and feed conversion rate of young Iranian and French Rainbow trout (Oncorhynchus mykiss). World Appl Sci J 21:1440–1444
Bernard DJ, Abuav-Nussbaum R, Horton TH, Turek FW (1999) Photoperiodic effects on gonadotropin-releasing hormone (GnRH) content and the GnRH-immunoreactive neuronal system of male Siberian Hamsters. Biol Reprod 60:272–276
Biswas AK, Takeuchi T (2003) Effects of photoperiod and feeding interval on food intake and growth rate of Nile tilapia (Oreochromis niloticus L.). Fish Sci 69:1010–1016
Biswas AK, Seoka M, Inoue Y, Takii K, Kuma H (2005) Photoperiod influences the growth, food intake, feed efficiency and digestibility of red sea bream (Pagrus major). Aquaculture 250:666–673
Boeuf G, Le Bail PY (1999) Does light have an influence on fish growth? Aquaculture 177:129–152
Borg B, Peute J, Reschke M, Van den HR (1987) Effects of photoperiod and temperature on testes, renal epithelium and pituitary gonadotropic cells of the three-spine stickleback, Gasterosteus aculeatus L. Can J Zool 65:14–19
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Cassone VM (1998) Melatonin’s role in vertebrate circadian rhythms. Chronobiol Int 15:457–473
Cohen H (1946) Effects of sex hormones on the development of the platyfish, Platypoecilus maculatus. Zoologica 31:121–128
Cruz EMV, Brown CL (2009) Influence of the photoperiod on growth rate and insulin-like growth factor-I gene expression in Nile tilapia Oreochromis niloticus L. J Fish Biol 75:130–141
Davie A, Mark JRP, Niall RB, Migaud H (2007) The role of seasonally altering photoperiod in regulating physiology in Atlantic cod (Gadus morhua). Part I Sexual maturation. Can J Fish Aquat Sci 64:84–97
Davies B, Bromage N, Swanson P (1999) The brain-pituitarygonadalxis of female rainbow trout Oncorhynchus mikiss: effects of photoperiod manipulation. Gen Comp Endocrinol 115:155–166
Davis SJ (2002) Photoperiodism: the coincidental perception of the season. Curr Biol 12:841–843
El-Sayed AF, Kawanna M (2004) Effects of photoperiod on the performance of farmed Nile tilapia Oreochromis niloticus: I. Growth, feed utilization efficiency and survival of fry and fingerlings. Aquaculture 231:393–402
El-Sayed AFM, Kawanna M (2007) Effects of photoperiod on growth and spawning efficiency of Nile tilapia (Oreochromis niloticus L.) broodstock in a recycling system. Aquac Res 28:1242–1247
Fielder DS, Bardsley WJ, Allan GL, Pankhurst PM (2002) Effect of photoperiod on growth and survival of snapper Pagrus auratus larvae. Aquaculture 211:135–150
Fiszebin A, Canepa M, Vazquez GR, Maggese C, Pandolfi M (2010) Photoperiodic modulation of reproductive physiology and behaviour in the cichlid fish Cichlasoma dimerus. Physiol Behav 99:425–432
Fraile B, Francisco J, Saez CA, Vicentini A, Gonzalez MPD, Ricardo P (1994) Effects of temperature and photoperiod on the Gambusia affinis holbrooki testis during the spermatogenesis period. Copia 1994:216–221
García-López Á, Pascual E, Sarasquete C, Martínez-Rodríguez G (2006) Disruption of gonadal maturation in cultured Senegalese sole Solea senegalensis Kaup by continuous light and/or constant temperature regimes. Aquaculture 261:789–798
Giannecchini LG, Massago H, Fernandes JBK (2012) Effects of photoperiod on reproduction of Siamese fighting fish Betta splendens. Rev Bras Zootec 41:821–826
Ginés R, Afonso JM, Argüello A, Zamorano MJ, López JL (2003) Growth in adult Ggilthead sea bream (Sparus aurata L) as a result of interference in sexual maturation by different photoperiod regimes. Aquac Res 34:73–83
Gines R, Afonso JM, Arguello A, Zamorano MJ, Lopez JL (2004) The effects of long-day photoperiod on growth, body composition and skin color in immature gilthead sea bream (Sparus aurata L.). Aquac Res 35:1207–1212
Hansen T, Karlsen O, Taranger GL, Hemre GI, Holm JC, Kjesbu O (2001) Growth, gonadal development and spawning time of Atlantic cod (Gadus morhua) reared under different photoperiod regimes. Aquaculture 203:51–67
Hau M (2001) Timing of breeding in variable environments tropical birds, as model systems. Horm Behav 40:281–290
Haynes JL (1995) Standardized classification of poeciliid development for life-history studies. Copeia 1995:147–154
Jourdan S, Fontaine P, Boujard T, Vandeloise E (2000) Influence of daylength on growth, heterogeneity, gonad development, sexual steroid and thyroid levels, and N and P budgets in Perca fluviatilis. Aquaculture 186:253–265
Kissil GW, Lupatsch I, Elizur A, Zohar Y (2001) Long photoperiod delayed spawning and increased somatic growth in gilthead seabream (Sparus aurata). Aquaculture 200:363–379
Lam TJ, Soh CL (1975) Effect of photoperiod on gonadal maturation in the Rabbitfish, Siganus canaliculatus Park, 1797. Aquaculture 5:407–410
Maitra K, Chattoraj A (2007) Role of photoperiod and melatonin in the regulation of ovarian functions in Indian carp Catla catla: basic information for future application. Fish Physiol Biochem 33:367–382
Migaud H, Davie A, Taylor JFT (2010) Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. Journal of Fish Biology 76: 27–68
Miranda LA, Strussmann CA, Somoza GM (2009) Effects of light and temperature conditions on the expression of GnRH and GtH genes and levels of plasma steroids in Odontesthes bonariensis females. Fish Physiol Biochem 35:101–108
Navarro FKSP, Navarro RD (2012) Importance of photoperiod in fish growth and reproduction. Revista Brasileira Reprod Animal 36:94–99
Navarro RD, De Sousa SC, Bizarro YWS, Da Silva RS, Pereira Navarro FKS (2015) Effects of photoperiod on somatic growth and gonadal development in male Nile tilapia. Acta Sci Technol 37:381–385
Rad F, Bozaoglu S, Gözükara SE, Karahan A, Kurt G (2006) Effects of different long- day photoperiods on somatic growth and gonadal development in Nile tilapia Oreochromis niloticus L. Aquaculture 255:292–300
Reynalte-Tataje D, Luz RK, Meurer S, Zaniboni-Filho EE, Nuñer APO (2002) Influence of photoperiod on growth and survival of post-larvae of Piracanjuba brycon orbignynaus (Valenciennes, 1849) (Osteichthyes, Characidae). Acta Sci Technol 24:439–443
Shahkar E, Dae-jung K, Mahmoud M, Hossein K, Hyeonho Y, Sungchul CB (2015) Effects of photoperiod manipulation on growth performance and hematological responses of juvenile caspian roach Rutilus rutilus caspicus. Fish Aquat Sci 18:51–56
Shan X, Xiao Z, Huang W, Dou S (2008) Effects of photoperiod on growth, mortality and digestive enzymes in miiuy croaker larvae and juveniles. Aquaculture 281:70–76
Smith BB, Walker KF (2004) Spawning dynamics of common carp in the River Murray, South Australia, shown by macroscopic and histological staging of gonads. J Fish Biol 64:336–354
Strussmann CA (1989) Basic studies on seed production of pejerrey Odontesthes bonariensis. PhD thesis. Tokyo University of Fisheries, Tokyo
Tavolga WN (1949) Embryonic development of the platyfish (Platypoecilus), the swordtail (Xiphophorus) and their hybrids. Bull Am Mus Nat Hist 94:161–230
Tavolga WN, Rugh R (1947) Development of the platyfish, Platypoecilus maculatus. Zoologica 32:1–15
Thorpe JE, Morgan RIG, Pretswell D, Higgins PJ (1988) Movements rhythms in juvenile Atlantic salmon, Salmo salar L. J Fish Biol 33:931–940
Tripple EA, Neil SRE (2003) Effects of photoperiod and light intensity on growth and activity of juvenile haddock (Melanogrammus aeglefinus). Aquaculture 217:633–645
Trotter AJ, Battaglene SC, Pankhurst PM (2003) Effects of photoperiod and light intensity on initial swim bladder inflation, growth and post-inflation viability in cultured striped trumpeter (Latris lineata) larvae. Aquaculture 224:141–158
Uribe MC, Harry JG, Víctor M (2014) Comparative testicular structure and spermatogenesis in bony fishes. Spermatogenesis. 4(3):e983400. https://doi.org/10.4161/21565562.2014.983400
Van Oordt GJ (1928) The duration of life of the spermatozoa in the fertilized female of Xiphophorus hellerii Regan. Tijdschr Nederlandsche Dierkund Vereen 1:77–80
Veras GC, Brabo MF, Dias JA, Abe HA, Nunes ZMP, Murgas LDS (2016) The effect of photoperiod and feeding frequency on larval of the Amazonian ornamental fish Pyrrhulina brevis (Steindachner, 1876). Aquac Res 47:797–803
Walton JC, Weil ZM, Nelson RJ (2011) Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol 32:303–319
Wolf LE (1931) The history of germ cells in the viviparous teleost Platypoecilus maculatus. J Morphol Physiol 52:115–153
Zhu D, Yang K, Gul Y, Song W, Zhang X, Wang W (2014) Effect of photoperiod on growth and gonadal development of juvenile Topmouth gudgeon (Pseudorasbora parva). Environ Biol Fish 97:147–156
Zutshi B, Singh A (2019) Impact of photoperiod as an environmental cue on growth and reproductive performance in the red eyed orange molly (Poecilia sphenops). Proc Zool Soc 1-7
Acknowledgments
The authors would like to thank the Department of Zoology, Bangalore University, Bengaluru and Dr. D. Seenappa (Chief Scientific Officer, Inland Fisheries Division, University of Agricultural, Hebbal, Bengaluru) for their continuous support during the conduct of present research work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 641 kb)
Rights and permissions
About this article
Cite this article
Singh, A., Zutshi, B. Photoperiodic effects on somatic growth and gonadal maturation in Mickey Mouse platy, Xiphophorus maculatus (Gunther, 1866). Fish Physiol Biochem 46, 1483–1495 (2020). https://doi.org/10.1007/s10695-020-00806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00806-8