Abstract
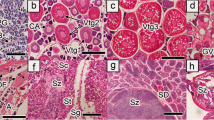
Bonefishes (Albula spp.) are classified within the superorder Elopomorpha, which is comprised of over 1000 species that share a unique leptocephalus larval stage. Bonefishes have a circum-tropical distribution, inhabiting inshore shallow water flats and gathering in presumptive nearshore pre-spawn aggregations (PSA) during spawning months. These fishes support economically important recreational fisheries and subsistence fisheries throughout their ranges, yet little is known regarding their reproductive biology. Analysis of oocyte development and nutrient composition, and sex and gonadotrophic hormone levels, was conducted on females sampled in Grand Bahama, Central Andros, and South Andros, The Bahamas, to assess their reproductive state. Fish collected from the flats habitats along all three islands exhibited four major reproductive phases (immature, developing, spawning capable, and regressing). In contrast, all females captured at presumptive PSA sites had eggs in the final stage of oocyte maturation, significantly higher levels of all reproductive hormones (17β-estradiol, testosterone, and LH), larger vitellogenic oocytes, and oocytes exhibiting germinal vesicle migration and germinal vesicle breakdown. In addition, monthly variability in hormone levels and spawning readiness between Grand Bahama and Andros PSAs suggest that peak spawning times may differ among regions. Fatty acid and free amino acid composition and profiles, with high proportions of docosahexaenoic acid, histidine, and taurine, suggest that these nutrients are not only relevant as energy reserves, but also help achieve buoyancy and osmoregulation of oocytes. This study expands upon our understanding of fish reproductive and developmental physiology, and indicates potential factors influencing the survival and recruitment of bonefishes.






Similar content being viewed by others
References
Adams AJ, Rehage JS, Cooke SJ (2019a) A multi-methods approach is essential for effective conservation and management of recreational flats fisheries. Environ Biol Fish 102(2):105–115
Adams AJ, Shenker JM, Jud ZR, Lewis JP, Carey E, Danylchuk AJ (2019b) Identifying pre-spawning aggregation sites for bonefish (Albula vulpes) in the Bahamas to inform habitat protection and species conservation. Environ Biol Fish 102:159–173
Alexander EC (1961) A contribution to the life history, biology and geographical distribution of bonefish, Albula vulpes (Linnaeus). Carlsberg Foundation, Copenhagen
Barber BJ (1996) Gametogenesis of eastern oysters, Crassostrea virginica (Gmelin, 1791) and Pacific oysters, Crassostrea gigas (Thunberg, 1793) in disease-endemic lower Chesapeake Bay. J Shellfish Res 15:285–290
Boucek RE, Lewis JP, Stewart BD, Jud ZR, Carey E, Adams AJ (2019) Measuring spatial use patterns and spawning site catchment areas of bonefish (Albula vulpes): using mark-recapture to inform habitat conservation. Environ Biol Fish 102(2):185–195
Bromage N, Jones J, Randall C, Thrush M, Davies B, Springate J, Duston J, Barker G (1992) Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 100(1–3):141–166
Cerdá J, Carrillo M, Zanuy S, Ramos J, de la Higuera M (1994) Influence of nutritional composition of diet on sea bass, Dicentrarchus labrax L., reproductive performance and egg and larval quality. Aquaculture 128:345–361
Colin, P. L., Sadovy, Y. J. and Domeier, M. L. 2003. Manual for the Study and Conservation of Reef Fish Spawning Aggregations. Society for the Conservation of Reef Fish Aggregations Special Publication No. 1 (Version 1.0), pp. 1-98+iii
Crabtree RE, Snodgrass D, Harnden CW (1997) Maturation and reproductive seasonality in bonefish, Albula vulpes, from the waters of the Florida Keys. Fish Bull 95:456–465
Danylchuk AJ, Cooke SJ, Goldberg TL, Suski CD, Murchie KJ, Danylchuk SE, Philipp DP (2011) Aggregations and offshore movements as indicators of spawning activity of bonefish (Albula vulpes) in the Bahamas. Mar Biol:1981–1999
Danylchuk AJ, Lewis J, Jud Z, Shenker J, Adams A (2019) Behavioral observations of bonefish (Albula vulpes) during prespawning aggregations in the Bahamas: clues to identifying spawning sites that can drive broader conservation efforts. Environ Biol Fish 102(2):175–184
Domeier ML (2012) Revisiting spawning aggregations: definitions and challenges. In: Sadovy de Mitcheson Y, Colin P (eds) Reef fish spawning aggregations: biology, research and management, Fish & Fisheries Series, vol 35. Springer, Dordrecht
El-Sayed A-FM (2014) Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev Aquac 6:241–255
Erisman BE, Cota-Nieto JJ, Moreno-Báez M, Aburto-Oropeza O (2017) Vulnerability of spawning aggregations of a coastal marine fish to a small-scale fishery. Mar Biol 164:5
Fedler T (2010) The economic impact of flats fishing in the Bahamas Report prepared for the Bahamian flats fishing alliance pp 20
Fedler T (2013) Economic impact of the Florida Keys flats fishery. Report prepared for Bonefish and Tarpon Trust
Fedler T (2014) 2013 economic impact of flats fishing in Belize. Report prepared for Bonefish and Tarpon Trust
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. Biol Chem 226:497–509
Furuita H, Ohta H, Unuma T, Tanaka H, Kagawa H, Suzuki N, Yamamoto T (2003) Biochemical composition of eggsin relation to egg quality in the Japanese eel, Anguilla japonica. Fish Physiol Biochem 29:37–46
Ganias K, Lowerre-Barbieri S (2018) Oocyte recruitment and fecundity type in fishes: refining terms to reflect underlying processes and drivers. Fish Fish 19:562–572
Hansen T, Karlsen Ø, Taranger GL, Hemre G-I, Holm JC, Kjesbu OS (2001) Growth, gonadal development and spawning time of Atlantic cod (Gadus morhua) reared under different photoperiods. Aquaculture 203:51–67
Holt G, Faulk CK, Schwarz MH (2007) A review of the larviculture of cobia Rachycentron canadum, a warm water marine fish. Aquaculture 268:181–187
Izquierdo MS, Fernández-Palacios H, Tacon AGJ (2001) Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197:25–42
Johannes RE (1978) Reproductive strategies of coastal marine fishes in the tropics. Environ Biol Fish 3:65–84
Lepage G, Roy C (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
Lowerre-Barbieri SK, Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3:52–70
Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Luck C, Mejri S, Lewis J, Wills PS, Riche M, Shenker J, Adams A, Ajemian MJ (2019) Seasonal and spatial changes in sex hormone levels and oocyte development of bonefish (Albula vulpes). Environ Biol Fish 102:209–219
Marty Y, Delaunay F, Moal J, Samain JF (1992) Changes in the fatty acid composition of Pecten maximus (L.) during larval development. J Exp Mar Biol Ecol 163:221–234
Mazurais D, Kjørsvik E, Wold PA, Politis S (2013) Biochemical, histological and molecular study of digestive tract development in European eel larvae (Anguilla anguilla) prior to exogenous feeding. Paper presented at the Aquaculture Europe, Trondheim, Norway
Mazzeo I, Peñaranda DS, Gallego V, Baloche S, Nourizadeh-Lillabadi R, Tveiten H, Dufour S, Asturiano JF, Weltzien FA, Pérez L (2014) Temperature modulates the progression of vitellogenesis in the European eel. Aquaculture 434:38–47
McKenzie DJ, Higgs DA, Dosanjh BS, Deacon G, Randall DJ (1998) Dietary fatty acid composition influences swimming performance in Atlantic salmon (Salmo salar) in seawater. Fish Physiol Biochem 19:111–122
Mejri S, Luck C, Tremblay R, Riche M, Adams A, Ajemian MJ, Shenker J, Wills PS (2019) Bonefish ( Albula vulpes) oocyte lipid class and fatty acid composition related to their development. Environ Biol Fish:1–12
Murchie KJ, Cooke SJ, Danylchuk AJ, Danylchuk SE, Goldberg TL, Suski CD, Philipp DP (2013) Movement patterns of bonefish (Albula vulpes) in tidal creeks and coastal waters of Eleuthera, the Bahamas. Fish Res 147:404–412
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Int J Dev Biol 38(2):217–229
Nagasawa T, Yonekura T, Nishizawa N, Kitts DD (2001) In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol Cell Biochem 225:29–34
Nancy J. Brown-Peterson, David M. Wyanski, Fran Saborido-Rey, Beverly J. Macewicz, Susan K. Lowerre-Barbieri, (2011) A Standardized Terminology for Describing Reproductive Development in Fishes. Marine and Coastal Fisheries 3 (1):52–70
Parrish CC (1999) Determination of total lipid, lipid classes, and fatty acids in aquatic samples. In: A MT, WBC (eds) Lipids in freshwater ecosystems. Springer Verlag, New York, pp 4–20
Pedersen BH (2003) Induced sexual maturation of the European eel Anguilla anguilla and fertilization of the eggs. Aquaculture 224:323–338
Qari AS, Moharram GS, Alowaidi AS (2013) Amino acids profile in gonads of the red sea fish Rhabdosargus sarba during breeding season. Int J Pharm Bio Sci 2:51–59
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rhodes JD, Breck O, Waagbo R, Bjerkas E, Sanderson J (2010) N-acetylhistidine, a novel osmolyte in the lens of Atlantic salmon (Salmo salar L.). Am J Phys Regul Integr Comp Phys 299:R1075–R1081
Rønnestad I, Fyhn HJ (1993) Metabolic aspects of free amino acids in developing marine fish eggs and larvae. Rev Fish Sci 1(3):239–259
Rønnestad I, Robertson R, Fyhn HJ (1996) Free amino acids and protein content in pelagic and demersal eggs of tropical marine fishes. In: DD MK, Eldridge M (eds) The Fish Egg. American Fisheries Society, Bethesda, pp 81–84
Rønquist Knutsen H (2015) Morphological development of wild leptocephalus larvae of the European eel (Anguilla anguilla). Norwegian University of Science and Technology, Trondheim
Sadovy de Mitcheson Y, Cornish A, Domeier M, Colin P, Russell M, Kenyon C, Lindeman K (2008) A Global Baseline for Spawning Aggregations of Reef Fishes. Conservation Biology 22 (5):1233–1244
Samaee SM, Estévez A, Giménez G, Lahnsteiner F (2009) Evaluation of quantitative importance of egg lipids and fatty acids during embryos and larvae development in marine pelagophil teleosts: with an emphasis on Dentex dentex. J Exp Zool A Ecol Genet Physiol 311:735–751
Sargent JR, Bell JG, Bell MV, Henderson RJ, Tocher DR (1995) Requirement criteria for essential fatty acids. J Appl Ichthyol 11:183–198
Sargent J, Tocher D, Bell J (2002) The lipids. Academic, New York
Sarih S, Djellata A, Roo J, Hernández-Cruz CM, Fontanillas R, Rosenlund G, Izquierdo M, Fernández-Palacios H (2019) Effects of increased protein, histidine and taurine dietary levels on egg quality of greater amberjack (Seriola dumerili, Risso, 1810). Aquaculture 499:72–79
Thorsen A, Fyhn HJ, Wallace R (1993) Free amino acids as osmotic effectors for oocyte hydration in marine fishes. In: Walther BT, Fyhn HJ (eds) Physiology and biochemistry of fish larval development. University of Bergen, Bergen, pp 94–98
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107
Vázquez R, González S, Rodriguez A, Mourente G (1994) Biochemical composition and fatty acid content of fertilized eggs, yolk sac stage larvae and first-feeding larvae of the Senegal sole (Solea senegalensis Kaup). Aquaculture 119(2–3):273–286
Wiegand MD (1996) Composition, accumulation and utilization of yolk lipids in teleost fish. Rev Fish Biol Fish 6:259–286
Wilson C, Scotto L, Scarpa J, Volety A, Laramore S, Haunert D (2005) Survey of water quality, oyster reproduction, and oyster health status in the St. Lucie Estuary. J Shellfish Res 24:157–165
Yanes-Roca C, Rhody N, Nystrom M, Main KL (2009) Effects of fatty acid composition and spawning season patterns on egg quality and larval survival in common Snook (Centropomus undecimalis). Aquaculture 287:335–340
Acknowledgments
We are grateful for the lodging and water access provided by East End Lodge, Andros South, and Hank’s Place, and for the extensive knowledge provided by Justin Lewis. The experimental protocol for this study received approval from Florida Atlantic University’s Institutional Animal Care and Use Committee (Animal Use Protocol #A16-34). HBOI-FAU’s IACUC committee follows the animal welfare guidelines in the National Research Councils “Guide for the Care and Use of Laboratory Animals, 8th Edition” National Academy Press Washington D.C. 2011. In addition, we follow the American Fisheries Society’s “Guidelines for the Use of Fishes in Research” American Fisheries Society, Bethesda, M.D. 2014. HBOI-FAU’s animal care facilities are accredited by American Association for the Accreditation of Laboratory Animal Care (AAALAC).
Funding
This study was financially supported by Bonefish & Tarpon Trust (BTT) and National Fish and Wildlife Foundation (NFWF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mejri, S., Luck, C., Wills, P.S. et al. Reproductive physiology of bonefishes (Albula spp.) across the Northwest Bahamas. Fish Physiol Biochem 46, 699–712 (2020). https://doi.org/10.1007/s10695-019-00743-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00743-1