Abstract
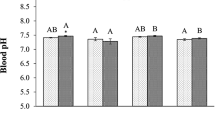
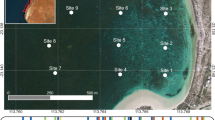
Estuaries are unstable ecosystems and can be changed by the environmental and anthropogenic impact. The Murray Estuary and Coorong were degraded by drought and low freshwater input in the last decade and therefore transformed into the largest hyper-saline lagoon in Australia. This study evaluates the physiological stress of two estuarine fish species (small-mouthed hardyhead Atherinosoma microstoma and Tamar goby Afurcagobius tamarensis) to the induced salinity change in captivity. The test fishes were collected from the Coorong and transported to the laboratory in the water from the Coorong. Each fish species was exposed to different levels of salinity, and a number of enzymes were assessed to measure the stress response of fish to salinity change. The activity of reactive oxygen species was significantly increased with the salinity change in both fish species compared with the fish in the control. Significant salinity effect on superoxide dismutase activity was observed on Tamar goby but not on small-mouthed hardyhead. Conversely, the impact of salinity on catalase activity was detected on small-mouthed hardyhead but not on Tamar goby. The study reveals that the induction of physical stress by salinity changes occurred in both Tamar goby and small-mouthed hardyhead despite the varying response of antioxidant enzymes between fish species. The study provides an insight into the understanding of physiological adaptation in estuarine fish to salinity change. The results could improve our knowledge on stress response and resilience of estuarine fish to hypo- and hyper-salinity stress.



Similar content being viewed by others
References
Abele D, Puntarulo S (2004) Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A: Mol Integr Physiol 138:405–415
Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J 21:143–152
An MI, Choi CY (2010) Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comp Biochem Physiol B: Biochem Mol Biol 155:34–42
An KW, Kim NN, Shin HS, Kil G-S, Choi CY (2010) Profiles of antioxidant gene expression and physiological changes by thermal and hypoosmotic stresses in black porgy (Acanthopagrus schlegeli). Comp Biochem Physiol A: Mol Integr Physiol 156:262–268
Barber SC, Mead RJ, Shaw PJ (2006) Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta Mol Basis Dis 1762:1051–1067
Beck MW et al (2001) The Identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 51:633–641
Blaber S (2002) `Fish in hot water’: the challenges facing fish and fisheries research in tropical estuaries. J Fish Biol 61:1–20
Brookes JD et al (2015) Fish productivity in the lower lakes and Coorong, Australia, during severe drought. Trans R Soc S Aust 139:189–215
Bucater L, Livore J, Noell C, Ye Q (2013) Temporal variation of larval fish assemblages of the Murray Mouth in prolonged drought conditions. Mar Freshw Res 64:932–937
Carocho M, Ferreira IC (2013) A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Doherty V, Ogunkuade O, Kanife U (2010) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in some selected fishes in Lagos, Nigeria. American–Eurasian J Agric and Environ Sci 7:359–365
Eckert J, Robinson R (1990) The fishes of the Coorong. S Aust Nat 65:4–31
Edgar GJ, Barrett NS, Graddon DJ, Last PR (2000) The conservation significance of estuaries: a classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biol Conserv 92:383–397
Ferguson GJ, Ward TM, Ye Q, Geddes MC, Gillanders BM (2013) Impacts of drought, flow regime, and fishing on the fish assemblage in Southern Australia’s largest temperate estuary. Estuar Coast 36:737–753
Ferreira M, Moradas-Ferreira P, Reis-Henriques M (2005) Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat Toxicol 71:39–48
Freire CA, Welker AF, Storey JM, Storey KB, Hermes‐Lima M (2011) Oxidative stress in estuarine and intertidal environments (temperate and tropical). In: Abele D, Vázquez-Medina JP, Zenteno-Savín T (eds) Oxidative stress in aquatic ecosystems. Wiley, New York, pp 41–57
Gillanders BM, Elsdon TS, Halliday IA, Jenkins GP, Robins JB, Valesini FJ (2011) Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review. Mar Freshw Res 62:1115–1131
Gonzalez R (2012) The physiology of hyper-salinity tolerance in teleost fish: a review. J Comp Physiol B 182:321–329
Halliwell B, Aruoma OI (1991) DNA damage by oxygen-derived species its mechanism and measurement in mammalian systems. FEBS Lett 281:9–19
Heink AE, Parrish AN, Thorgaard GH, Carter PA (2013) Oxidative stress among SOD-1 genotypes in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 144:75–82
Hossain MA, Ye Q, Leterme SC, Qin J (2016) Spatial and temporal changes of three prey-fish assemblage structure in a hypersaline lagoon: the Coorong, South Australia. Mar Freshw Res. doi:10.1071/MF15212
Kingsford RT, Walker KF, Lester RE, Young WJ, Fairweather PG, Sammut J, Geddes MC (2011) A Ramsar wetland in crisis–the Coorong, Lower Lakes and Murray Mouth, Australia. Mar Freshw Res 62:255–265
Lackner R (1998) “Oxidative stress” in fish by environmental pollutants. In: Fish Ecotoxicology. Springer, p 203–224
Lavado R, Shi D, Schlenk D (2012) Effects of salinity on the toxicity and biotransformation of l-selenomethionine in Japanese medaka (Oryzias latipes) embryos: mechanisms of oxidative stress. Aquat Toxicol 108:18–22
Li E, Chen L, Zeng C, Yu N, Xiong Z, Chen X, Qin JG (2008) Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 274:80–86
Liepelt A, Karbe L, Westendorf J (1995) Induction of DNA strand breaks in rainbow trout Oncorhynchus mykiss under hypoxic and hyperoxic conditions. Aquat Toxicol 33:177–181
Lintermans M (2007) Fishes of the Murray-Darling Basin: an introductory guide. MDBC Publication No. 10/07, Murray-Darling Basin Authority, Canberra
Liu Y, Wang W-N, Wang A-L, Wang J-M, Sun R-Y (2007) Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture 265:351–358
Lui LC (1969) Salinity tolerance and osmoregulation of Taeniomembers microstomus (Gunther, 1861) (Pisces: Mugiliformes: Atherinidae) from Australian salt lakes. Mar Freshw Res 20:157–162
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Ma ZH, Zheng PL, Guo HY, Jiang SG, Qin JG, Zhang DC, Liu XL (2016) Salinity regulates antioxidant enzyme and Na+K+-ATPase activities of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquac Res 47:1481–1487
Madeira D, Narciso L, Cabral H, Vinagre C, Diniz M (2013) Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp Biochem Physiol A: Mol Integr Physiol 166:237–243
Martinez-Alvarez R, Hidalgo M, Domezain A, Morales A, García-Gallego M, Sanz A (2002) Physiological changes of sturgeon Acipenser naccarii caused by increasing environmental salinity. J Exp Biol 205:3699–3706
Matoo OB, Ivanina AV, Ullstad C, Beniash E, Sokolova IM (2013) Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp Biochem Physiol A: Mol Integr Physiol 164:545–553
McNeil D, Westergaard S, Cheshire K, Noell C, Ye Q (2013) Effects of hyper-saline conditions upon six estuarine fish species from the Coorong and Murray Mouth. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2009/000014-4. SARDI Research Report Series No. 700
Noell CJ, Ye Q, Short DA, Bucater L, Wellman NR (2009) Fish assemblages of the Murray Mouth and Coorong region, South Australia, during an extended drought period. CSIRO Water for a Healthy Country National Research Flagship and South Australian Research and Development Institute (Aquatic Sciences)
Paital B, Chainy G (2010) Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp Biochem Physiol C: Toxicol Pharmacol 151:142–151
Parihar M, Dubey A (1995) Lipid peroxidation and ascorbic acid status in respiratory organs of male and female freshwater catfish Heteropneustes fossilis exposed to temperature increase. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 112:309–313
Paton D (2010) At the end of the river: the Coorong and lower lakes. ATF Press, Hindmash
Roche H, Bogé G (1996) Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar Environ Res 41:27–43
Ross SW, Dalton DA, Kramer S, Christensen B (2001) Physiological (antioxidant) responses of estuarine fishes to variability in dissolved oxygen. Comp. Biochem. Physiol. C: Toxicol. Pharmacol 130:289–303
Rudneva I (1999) Antioxidant system of black sea animals in early development. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 122:265–271
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual+ Cold Spring Harbor. Cold Spring Harbor Laboratory Press, New York
Sinha AK et al (2015) Nutritional status as the key modulator of antioxidant responses induced by high environmental ammonia and salinity stress in European sea bass (Dicentrarchus labrax). PLoS One 10:e0135091
Smith A (1976) Catalase in fish red blood cells. Comp Biochem Physiol B: Comp Biochem 54:331–332
Telesh IV, Khlebovich VV (2010) Principal processes within the estuarine salinity gradient: a review. Mar Pollut Bull 61:149–155
Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Wdziȩczak J, Zaleśna G, Wujec E, Peres G (1982) Comparative studies on superoxide dismutase, catalase and peroxidase levels in erythrocytes and livers of different freshwater and marine fish species. Comp Biochem Physiol B: Comp Biochem 73:361–365
Webster IT (2010) The hydrodynamics and salinity regime of a coastal lagoon–the Coorong, Australia-Seasonal to multi-decadal timescales. Estuar Coast Shelf Sci 90:264–274
Wedderburn SD, Walker KF, Zampatti BP (2008) Salinity may cause fragmentation of hardyhead (Teleostei: Atherinidae) populations in the River Murray, Australia. Mar Freshw Res 59:254–258
Wedderburn SD, Bailey CP, Delean S, Paton DC (2016) Population and osmoregulatory responses of a euryhaline fish to extreme salinity fluctuations in coastal lagoons of the Coorong, Australia. Estuar Coast Shelf Sci 168:50–57
Wilhelm Filho D, Giulivi C, Boveris A (1993) Antioxidant defences in marine fish—I. Teleosts. Comp Biochem Physiol 106:409–413
Williams W, Boulton A, Taaffe R (1990) Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197:257–266
Zampatti BP, Bice CM, Jennings PR (2010) Temporal variability in fish assemblage structure and recruitment in a freshwater-deprived estuary: the Coorong. Australia Mar Freshw Res 61:1298–1312
Acknowledgments
We would like to thank David Short, Neil Wellman, Shaun Henderson, Leslie Morrison, Kathy Schuller and Sarmad Al-Asadi for their assistance in the field and in laboratory. This project was carried out under a Flinders University Animal Welfare Committee Project E409. This project was partially supported by Flinders International Postgraduate Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hossain, M.A., Aktar, S. & Qin, J.G. Salinity stress response in estuarine fishes from the Murray Estuary and Coorong, South Australia. Fish Physiol Biochem 42, 1571–1580 (2016). https://doi.org/10.1007/s10695-016-0241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0241-3