Abstract
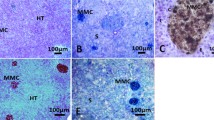
In the catfish Heteropneustes fossilis, the anterior kidney is a hemopoietic tissue which surrounds the adrenal homologues, interrenal (IR) and chromaffin tissues corresponding to the adrenal cortical and adrenal medulla of higher mammals. The IR tissue is arranged in cell cords around the posterior cardinal vein (PCV) and its tributaries and secretes corticosteroids. The chromaffin tissue is scattered singly or in nests of one or more cells around the epithelial lining of the PCV or blood capillaries within the IR tissue. They are ferric ferricyanide-positive. Leukemia-inhibitory factor (LIF)-like reactivity was noticed in the lining of the epithelium of the IR cell cords and around the wall of the PCV and blood capillaries. No staining was observed in the hemopoietic cells. IL-1β- and TNF-α-like immunoreactivity was seen in certain cells in the hemopoietic tissue but not in the IR region. Macrophages were identified with mammalian macrophage-specific MAC387 antibodies and are present in the hemopoietic mass but not in the IR tissue. Pigments accumulate in the hemopoietic mass as melano-macrophage centers (MMCs) and are PAS-, Schmorl’s- and Perls’-positive. The pigments contain melanin (black), hemosiderin (blue) and lipofuscin/ceroid (oxidized lipid, yellowish tan), as evident from the Perls’ reaction. The MMCs were TUNEL-positive as evident from FITC fluorescence, indicating their apoptotic nature. The MMCs showed significant seasonal variation with their density increasing to the peak in the postspawning phase. Melanins were characterized spectrophotometrically for the first time in fish anterior kidney. The predominant form is pheomelanin (PM), followed by eumelanin (EM) and alkali-soluble melanin (ASM). Melanins showed significant seasonal variations with the level low in the resting phase and increasing to the peak in the postspawning phase. Under in vitro conditions, lipopolysaccharide (10 µg/mL) treatment increased significantly the levels of PM and EM levels both at 16 and at 32 h and the ASM level at 32 h. On the other hand, the synthetic glucocorticoid dexamethasone (100 nM) decreased significantly the levels of EM, PM and ASM time-dependently. The results indicate that the anterior kidney is an important site of immune–endocrine interaction.










Similar content being viewed by others
References
Abdel-Aziz EH, Abdu SBS, Ali TE, Fouad HF (2010a) Haemopoiesis in the head kidney of tilapia, Oreochromis niloticus (Teleostei: Cichlidae): a morphological (optical and ultra structural) study. Fish Physiol Biochem 36:323–336
Abdel-Aziz EH, Ali TE, Abdu SBS, Fouad HP (2010b) Chromaffin cells and interrenal tissue in the head kidney of the grouper, Epinephilus tauvina (Teleostei, Serranidae): a morphological (optical and ultra structural) study. J Appl Ichthyol 26:522–527
Abe T, Mikekado T, Haga S, Kisara Y, Watanabe K, Kurokawa T, Suzuki T (2007) Identification, cDNA cloning, and mRNA localization of a zebrafish ortholog of leukemia inhibitory factor. Comp Biochem Physiol B 147:38–44
Abelli L, Gallo VP, Civinini A, Mastrolia L (1996) Immunohistochemical and ultrastructural evidence of adrenal chromaffin cell subtypes in Sea Bass Dicentrarchus labrax (L.). Gen Comp Endocrinol 102:113–122
Agius C (1985) The melano-macrophage centers of fish: a review. In: Manning MJ, Tatner MF (eds) Fish Immunology. Academic Press, London, pp 85–104
Agius C, Roberts RJ (2003) Melano-macrophage centres and their role in fish pathology. J Fish Dis 26:499–509
Auernhammer CJ, Melmed S (2000) Leukemia-inhibitory factor- neuroimmune modulator of endocrine function. Endocr Rev 21:313–345
Balamurugan S, Deivasigamani B, Kumaran S, Sakthivel M, Rajsekar T, Priyadharsini P (2012) Melanomacrophage centres aggregation in P. lineatus spleen as bio-indicator of environmental change. Asian Pac J Trop Dis 12:1–4
Balm PHM, Lieshout E, Lokate J, Bonga SEW (1995) Bacterial lipopolysaccharide (LPS) and interleukin 1 (IL-1) exert multiple physiological effects in the tilapia Oreochromis mossambicus (Teleostei). J Comp Physiol 165:85–92
Bamberger AM, Schulte HM, Wullbrand A, Jung R, Beil FU, Bamberger CM (2000) Expression of leukemia inhibitory factor (LIF) and LIF receptor (LIF-R) in the human adrenal cortex: implications for steroidogenesis. Mol Cell Endocrinol 162:145–149
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, London
Besseau L, Faliex E (1994) Resorption of unemitted gametes in Lithognathus mormyrus (Sparidae: Teleostei): a possible synergic action of somatic and immune cells. Cell Tissue Res 276:123–132
Blazer VS (2002) Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol Biochem 26:85–110
Bornstein SR, Rutkowski H, Vrezas I (2004) Cytokines and steroidogenesis. Mol Cell Endocrinol 215:135–141
Braun-Nesje R, Kaplan G, Seljelid R (1982) Rainbow trout macrophages in vitro: morphology and phagocytic activity. Dev Comp Immunol 6:281–291
Butler DG (1973) Structure and function of the adrenal gland of fishes. Am Zool 13:839–879
Castellana B, Iliev DB, Sepulcre MP, Mackenzie S, Goetz FW, Mulero V, Planas JV (2008) Molecular characterization of interleukin-6 in the gilthead seabream (Sparus aurata). Mol Immunol 45:3363–3370
Chesnokova V, Melmed S (2000) Leukemia Inhibitory factor mediates the hypothalamic pituitary adrenal axis response to inflammation. Endocrinology 141:4032–4040
Chester J, Ingleton PM, Phillips JG (1986) Fundamentals of comparative vertebrate endocrinology. Plenum Press, New York, pp 95–120
Civinini A, Padula D, Gallo VP (2001) Ultrastructure and histochemical study on the interrenal cells of the male stickleback, Gasterosteus aculeatus teleostea, in relation to the reproductive annual cycle. J Anat 199:303–316
Crivellato E, Civinini A, Gallo VP (2006) Chromaffin cells in the adrenal Homolog of Aphanius fasciatus (Teleost fish) express Piecemeal degranulation in response to osmotic stress: a hint for a conservative evolutionary process. Anat Rec A Discov Mol Cell Evol Biol 288:1077–1086
Datta S, Ghosh D, Saha DR, Bhattacharaya S, Mazumder S (2009) Chronic exposure to low concentration of arsenic is immunotoxic to fish: role of head kidney macrophage as biomarkers of arsenic toxicity to Clarias batrachus. Aquat Toxicol 92:86–94
Diaz-Satizabal L, Magor BG (2015) Isolation and cytochemical characterization of melanomacrophages and melanomacrophage clusters from goldfish (Carassius auratus L). Dev Comp Immunol 48:221–228
Ellis AE (1977) The leucocytes of fish: a review. J Fish Biol 11:453–491
Engelsma MY, Huising MO, Van Muiswinkel WB, Flik G, Kwang J, Savelkoul HFJ, Verburg-van Kemenade LBM (2002) Neuroendocrine-immune interactions in fish: a role for interleukin-1. Vet Immunol Immunopathol 87:467–479
Fabbri E, Capuzzo A, Moon TW (1998) The role of circulating catecholamines in the regulation of fish metabolism: a overview. Comp Biochem Physiol C 120:177–192
Fan R, Yang G, Dong C (2010) Study of hair melanins in various hair color Alpaca (Lama pacos). Asian-Aust J Anim Sci 23:444–449
Fange R (1986) Physiology of haemopoiesis. In: Nilsson S, Holmgren S (eds) Fish physiology: recent advance. Groom Helm, London, pp 1–23
Fast MD, Johnson SC, Jones SRM (2007) Differential expression of the pro-inflammatory cytokines IL-1b-1, TNFa-1 and IL-8 in vaccinated pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon juveniles. Fish Shellfish Immunol 22:403–407
Fijan N (2002) Composition of main haematopoietic compartments in normal and bled channel catfish. J Fish Biol 60:1142–1154
Fishelson L (2006) Cytomorphological alterations of the thymus, spleen, head-kidney, and liver in cardinal fish (Apogonidae, Teleostei) as bioindicators of stress. J Morphol 267:57–69
Fournie JW, Summers JK, Courtney LA, Engle VD (2001) Utility of splenic macrophage aggregates as an indicator of fish exposure to degraded environments. J Aquat Anim Health 13:105–116
Franco-Belussi L, de Oliveira C (2011) Lipopolysaccharides induce changes in the visceral pigmentation of Eupemphix nattereri (Anura: Leiuperidae). Zoology 114:298–305
Franco-Belussi L, de Lauro Castrucci AM, de Oliveira C (2013) Response of melanocytes and melanomacrophages of Eupemphix nattereri (Anura: Leiuperidae) to Nle4, D-Phe7-α-melanocyte stimulating hormone and lipopolysaccharides. Zoology 116:316–324
Gallo VP, Civinini A, Mastrolia L, Leitner G, Porta S (1993) Cytological and biochemical studies on chromaffin cells in the head kidney of Gasterosteus aculeatus (Teleostei, Gasterosteidae). Gen Comp Endocrinol 92:133–142
Gonzalez-Hernandez JA, Bornstein SR, Ehrhart-Bornstein M, Gschwend JE (1995) IL-1 is expressed in human adrenal gland in vivo. Possible role in a local immune-adrenal axis. Clin Exp Immunol 99:137–141
Guthrie HD, Grimes RW, Cooper BS, Hammond JM (1995) Follicular atresia in pigs: measurement and physiology. J Anim Sci 73:2834–2844
Guzman C, Hernandez-Bello R, Morales-Montor J (2010) Regulation of steroidogenesis in reproductive, adrenal and neural tissues by cytokines. Open Neuroendocrinol J 3:161–169
Herraez MP, Zapata AG (1991) Structural characterization of the melano-macrophage centres (MMC) of goldfish Carassius auratus. Eur J Morphol 29:89–102
Holland JW, Pottinger TG, Secombes CJ (2002) Recombinant interleukin-1 activates the hypothalamic–pituitary–interrenal axis in rainbow trout, Oncorhynchus mykiss. J Endocrinol 175:261–267
Hughes FM Jr, Gorospe WC (1991) Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology 129:2415–2422
Iliev DB, Roach JC, Mackenzie S, Planas JV, Goetz FW (2005) Endotoxin recognition: in fish or not in fish? FEBS Lett 579:6519
Ito S, Wakamatsu K, Ozeki H (2000) Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res 13:103–109
Janz DM, Kraak GVD (1997) Suppression of apoptosis by gonadotropin, 17β-estradiol, and epidermal growth factor in rainbow trout preovulatory ovarian follicles. Gen Comp Endocrinol 105:186–193
Jordanova M, Miteva N, Rocha E (2008) A qualitative and quantitative study of the hepatic pigmented macrophage aggregates during the breeding cycle of Ohrid Trout, Salmo letnica Kar. (Teloestei, Salmonidae). Microsc Res Tech 71:822–830
Jordanova M, Rocha MJ, Rebok K, Rocha E (2012) Changes in the amount of kidney pigmented macrophage aggregates throughout the breeding cycle of female Ohrid trout, Salmo letnica Kar. (Teleostei, Salmonidae). Microsc Res Tech 75:176–181
Kemenade BMLV, Stolte EH, Metz JR, Chadzinska M (2009) Neuroendocrine-immune interactions in teleost fish. Fish Neuroendocronol 28:313–365
Kemenade BMLV, Ribeiro CMS, Chadzinska M (2011) Neuroendocrine–immune interaction in fish: differential regulation of phagocyte activity by neuroendocrine factors. Gen Comp Endocrinol 172:31–38
Kumar R, Joy KP (2015) Melanins as biomarkers of ovarian follicular atresia in the catfish Heteropneustes fossilis: biochemical and histochemical characterization, seasonal variation and hormone effects. Fish Physiol Biochem 41:761–772
Lei L, Tzekov R, Tang S, Kaushal S (2012) Accumulation and autofluorescence of phagocytized rod outer segment material in macrophages and microglial cells. Mol Vis 18:103–113
Lofts B, Bern HA (1972) Functional morphology of steroidogenic tissue. Idler DR (Edn) Steroids in non-mammalian vertebrates. Academic Press, New York, pp 37–125
Mastrolia L, Gallo VP, La Marca A (1984) The adrenal chromaffin cells of Salmo gairdneri Richardson (Teleostei, Salmonidae). J Anat 138:503–511
Matsche MA, Grizzle JM (1999) Early changes in pigmented macrophages in head kidney of channel catfish infected with Aeromonas hydrophila. J Aquat Anim Health 11:253–261
Meseguer J, Lopez-Ruiz A, Esteban MA (1994) Melano-macrophages of the seawater teleosts, sea bass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata): morphology, formation and possible function. Cell Tissue Res 277:1–10
Metz JR, Huising MO, Leon K, Vernurg-van Kemenade BML, Flik G (2006) Central and peripheral interleukin-1β and interleukin-1 receptor expression and their role in the stress response of common carp, Cyprinus carpio L. J Endocrinol 191:25–35
Milano EG, Basari F, Chimenti C (1997) Adrenocortical and adrenomedullary homologs in eight species of adult and developing teleosts: morphology, histology and immunohistochemistry. Gen Comp Endocrinol 108:483–496
Montpetit CJ, Perry SF (2002) Adrenergic regulation of catecholamine secretion from trout (Oncorhynchus mykiss) chromaffin cells. J Endocrinol 173:187–197
Mulero I, Sepulcre MP, Roca FJ, Meseguer J, Garcia-Ayala A, Mulero V (2008) Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Dev Comp Immunol 32:1151–1159
Nandi J (1962) The structure of the interrenal gland in teleost fishes. Univ Calif Publ Zool 65:129–212
Nascimento DS, Pereira PJB, Reis MIR, Vale A, Zou J, Silva MT, Secombes CJ, Santos NMS (2007) Molecular cloning and expression analysis of sea bass (Dicentrarchus labrax L.) tumor necrosis factor-α (TNF-α). Fish Shellfish Immunol 23:701–710
Ozeki H, Ito S, Wakamatsu K, Hirobe T (1995) Chemical characterization of hair melanins in various coat-color mutants of mice. J Invest Dermatol 105:361–366
Pelegrin P, Chaves-Pozo E, Mulero V, Meseguer J (2004) Production and mechanism of secretion of interleukin-1β from the marine fish gilthead seabream. Dev Comp Immunol 28:229–237
Press CM, Evensen O (1999) The morphology of the immune system in teleost fishes. Fish Shellfish Immunol 9:309–318
Qin QW, Ototake M, Noguchi K, Soma G, Yokomizo Y, Nakanishi T (2001) Tumor necrosis factor alpha (TNFα)-like factor produced by macrophages in rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol 11:245–256
Reid SG, Bernier NJ, Perry SF (1998) The adrenergic stress response in fish: control of catecholamine storage and release. Comp Biochem Physiol C 120:1–27
Ribeiro HJ, Procopio MS, Gomes JMM, Vieira FO, Russo RC, Balzuweit K, Chiarini- Garcia H, Casrro ACS, Rizzo E, Correa JD (2011) Functional dissimilarity of melano-macrophage centres in the liver and spleen from females of the teleost fish Prochilodus argenteus. Cell Tissue Res 346:417–425
Rocha RM, Santes HS, Vecentini CA, Cruz C (2001) Structural and ultrastructural characteristics of interrenal gland and chromaffin cell of matrinxa. Brycon cephalus Gunther 1869 (Teleostei-Characidae). Anat Histol Embryol 30:351–355
Romano N, Picchietti S, Taverne-Thiele JJ, Taverne N, Abelli L, Mastrolia L, Kemenade BMLV, Rombout JHWM (1998) Distribution of macrophages during fish development: an immunohistochemical study in carp (Cyprinus carpio L.). Anat Embryol 198:31–41
Sailendri K, Muthukkaruppan VR (1975) Morphology of lymphoid organ in a cichlid teleost, Tilapia mossambica (Peters). J Morphol 147:109–122
Santos HB, Thome RG, Arantes FP, Sato Y, Bazzoli N, Rizzo E (2008) Ovarian follicular atresia is mediated by heterophagy, autophagy, and apoptosis in Prochilodus argenteus and Leporinus taeniatus (Teleostei: Characiformes). Theriogenology 70:1449–1460
Sarmento A, Marques F, Ellis AE, Afonso A (2004) Modulation of the activity of sea bass (Dicentrarchus labrax) head-kidney macrophages by macrophage activating factor (s) and lipopolysaccharide. Fish Shellfish Immunol 16:79–92
Secombes CJ, Fletcher TC (1992) The role of phagocytes in the protective mechanisms of fish. Ann Rev Fish Dis 2:53–71
Seljelid R, Eskeland T (1993) The biology of macrophages: I general principles and properties. Eur J Haematol 51:267–275
Sorensen KK, Sveinbjornsson B, Dalmo RA, Smedsrod B, Bertheussen K (1997) Isolation, cultivation and characterization of head kidney macrophages from Atlantic cod, Gadus morhua L. J Fish Dis 20:93–107
Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, Atkins RC, Nikolic- Paterson DJ (1997) Intrinsic renal cells are the major source of interleukins-1β synthesis in normal and diseased rat kidney. Nephrol Dial Transplant 12:1109–1115
Tilly JL, Kowalski KI, Johnson AL, Hsueh AJW (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 129:2799–2801
Tkachenko IV, Jaaskelainen T, Jaaskelainen J, Palvimo JJ, Voutilainen R (2011) Interleukins 1α and 1β as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids 76:1103–1115
Ucuncu SI, Cakıcı O (2009) Atresia and apoptosis in preovulatory follicles in the ovary of Danio rerio (zebrafish). Turk J Fish Aquat Sci 9:215–221
Vermeulen GJ, Lambert JGD, Teitsma CA, Zandbergen MA, Goos HJT (1995) Adrenal tissue in the male African catfish, Clarias gariepinus: localization and steroid hormone secretion. Cell Tissue Res 280:653–657
Vigliano FA, Bermudez R, Quiroga IM, Nieto JM (2006) Evidence for melano-macrophage centres of teleost as evolutionary precursors of germinal centres of higher vertebrates: an immunohistochemical study. Fish Shellfish Immunol 21:467–471
Wolke RE (1992) Piscine macrophage aggregate, a review. Ann Rev Fish Dis 2:91–108
Wood AW, Van Der Kraak GJ (2001) Apoptosis and ovarian function: novel perspectives from the teleosts. Biol Reprod 64:264–271
Woods AM, McIlmoil CJ, Rankin EN, Packer AA, Stevens JC, Macievic JA, Brown AB, Porter JP, Judd AM (2008) Leukemia inhibitory factor protein and receptors are expressed in the bovine adrenal cortex and increase cortisol and decrease adrenal androgen release. Domest Anim Endocrinol 35:217–230
Zapata A, Diez B, Cejalvo T, Gutiérrez-de Frías C, Cortés A (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20:126–136
Zou J, Holland J, Pleguezuelos O, Cunningham C, Secombes CJ (2000) Factors influencing the expression of interleukin-1β in cultured rainbow trout (Oncorhynchus mykiss) leucocytes. Dev Comp Immunol 2:575–582
Zuasti A, Jara JR, Ferrer C, Solano F (1989) Occurrence of melanin granules and melanosynthesis in the kidney of Sparus auratus. Pigment Cell Res 2:93–99
Acknowledgments
R.K. is a recipient as senior research fellow of Rajiv Gandhi National Fellowship of UGC, New Delhi, which is gratefully acknowledged. This work was partly supported by the UGC-CAS Programme (No. 4197) to Department of Zoology, BHU. Our thanks are due to Prof. B. Lal, Department of Zoology, BHU, for the photomicrography facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Joy, K.P. & Singh, S.M. Morpho-histology of head kidney of female catfish Heteropneustes fossilis: seasonal variations in melano-macrophage centers, melanin contents and effects of lipopolysaccharide and dexamethasone on melanins. Fish Physiol Biochem 42, 1287–1306 (2016). https://doi.org/10.1007/s10695-016-0218-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0218-2