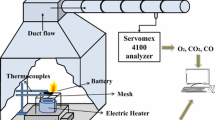
Abstract
Semi-solid lithium-ion flow battery (SSLFB) is a promising candidate in the field of large-scale energy storage. However, as a key component of SSLFB, the slurry presents a great fire hazard due to the extremely flammable electrolyte content in the slurry as high as 70 wt%–95 wt%. To evaluate the fire risk of SSFLB, the combustion experiments of electrolyte and slurry were conducted using cone calorimeter, and the critical fire parameters such as heat release rate (HRR), mass loss rate (MLR), and gas production were analyzed. This study firstly compared the combustion characteristics of electrolytes with the addition of different lithium salts (LiPF6 and LiTFSI). The results showed that the peak HRR (pHRR) and peak MLR (pMLR) of LiTFSI-based electrolyte were reduced by 30.3% and 33.2%, respectively, compared with LiPF6-based electrolyte. Also, LiTFSI-based electrolyte possessed a relatively lower toxic hazard. Overall, LiTFSI could reduce the fire hazard of the electrolyte compared with LiPF6. Then, the combustion behaviour of slurries containing different electrode materials (Li4Ti5O12, LiNi0.8Co0.1Mn0.1O2, LiFePO4, and Graphite) was investigated. It was observed that the splashing occurred in the early stage combustion of slurries. The splashing of S-LTO and S-LFP was relatively violent, while only sporadic splashing occurred for S-NCM and S-graphite. Based on the pHRR and pMLR test results, the order of fire risk of the four slurries is determined as S-LTO > S-LFP > S-NCM > S-Graphite. The pHRR and pMLR of slurries other than S-LTO are lower than that of electrolyte, thus their fire risk is lower than electrolyte. The results of this study can provide a reference for the fire hazard evaluation and safety improvement of the SSFLB system.















Similar content being viewed by others
References
Narayanan TM, Zhu YG, Gençer E, McKinley G, Shao-Horn Y (2021) Low-cost manganese dioxide semi-solid electrode for flow batteries. Joule 5:2934–2954. https://doi.org/10.1016/j.joule.2021.07.010
Wang Q, Mao B, Stoliarov SI, Sun J (2019) A review of lithium ion battery failure mechanisms and fire prevention strategies progress in energy and combustion. Science 73:95–131. https://doi.org/10.1016/j.pecs.2019.03.002
Hopkins BJ, Smith KC, Slocum AH, Chiang YM (2015) Component-cost and performance based comparison of flow and static batteries. J Power Sources 293:1032–1038. https://doi.org/10.1016/j.jpowsour.2015.06.023
Li L, Kim S, Wang W, Vijayakumar M, Nie Z, Chen B, Zhang J, Xia G, Hu J, Graff G, Liu J, Yang Z (2011) A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv Energy Mater 1:394–400. https://doi.org/10.1002/aenm.201100008
Rubio-Garcia J, Cui J, Parra-Puerto A, Kucernak A (2020) Hydrogen/vanadium hybrid redox flow battery with enhanced electrolyte concentration. Energy Storage Mater 31:1–10. https://doi.org/10.1016/j.ensm.2020.05.031
Zhang X, Li W, Chen H (2021) High-capacity CuSi2P3-based semisolid anolyte for redox flow batteries. ACS Appl Mater Interfaces 13:40552–40561. https://doi.org/10.1021/acsami.1c09590
Duduta M, Ho B, Wood VC, Limthongkul P, Brunini VE, Carter WC, Chiang YM (2011) Semi-solid lithium rechargeable flow battery. Adv Energy Mater 1:511–516. https://doi.org/10.1002/aenm.201100152
Chen H, Lu YC (2016) A high-energy-density multiple redox semi-solid-liquid flow battery. Adv Energy Mater 6:1502183. https://doi.org/10.1002/aenm.201502183
Lacroix R, Biendicho JJ, Mulder G, Sanz L, Flox C, Morante JR, Da Silva S (2019) Modelling the rheology and electrochemical performance of Li4Ti5O12 and LiNi1/3Co1/3Mn1/3O2 based suspensions for semi-solid flow batteries. Electrochim Acta 304:146–157. https://doi.org/10.1016/j.electacta.2019.02.107
Chen H, Liu Y, Zhang X, Lan Q, Chu Y, Li Y, Wu Q (2021) Single-component slurry based lithium-ion flow battery with 3D current collectors. J Power Sources 485:229319. https://doi.org/10.1016/j.jpowsour.2020.229319
Pan S, Zhang H, Xing C, Yang L, Su P, Bi J, Zhang S (2021) Ultrahigh-capacity semi-solid SiOx anolytes enabled by robust nanotube conductive networks for Li-ion flow batteries. J Power Sources. https://doi.org/10.1016/j.jpowsour.2021.230341
Wang Q, Jiang L, Yu Y, Sun J (2019) Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 55:93–114. https://doi.org/10.1016/j.nanoen.2018.10.035
Ribière P, Grugeon S, Morcrette M, Boyanov S, Laruelle S, Marlair G (2012) Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ Sci 5:5271–5280. https://doi.org/10.1039/c1ee02218k
Su P, Zhang H, Yang L, Xing C, Pan S, Lu W, Zhang S (2022) Effects of conductive additives on the percolation networks and rheological properties of LiMn0.7Fe0.3PO4 suspensions for lithium slurry battery. Chem Eng J. https://doi.org/10.1016/j.cej.2021.133203
Shouding LI, Yan LI, Jie TI, Yuming ZH, Min YA, Jun LU, Yuancheng CA (2020) Current status and emerging trends in the safety of Li-ion battery energy storage for power grid applications. Energy Storage Sci Technol 9:1505–1516. https://doi.org/10.19799/j.cnki.2095-4239.2020.0111
Zhang W, Chen X, Chen Q, Ding C, Liu J, Chen M, Wang J (2015) Combustion calorimetry of carbonate electrolytes used in lithium ion batteries. J Fire Sci 33:22–36. https://doi.org/10.1177/0734904114550789
Huang Q, Chen H, Zheng K, Zhu K, Liu C (2021) Comparison of oxygen consumption calorimetry and thermochemistry theory on quantitative analysis of electrolyte combustion characteristics. Case Studies Thermal Eng. https://doi.org/10.1016/j.csite.2021.101085
Chen MY, Xiao R, Zhao LY, Weng JW, Ouyang DX, Chen QP, Yao JJ, Wang J (2022) Experimental study on the combustion characteristics of carbonate solvents under different thermal radiation by cone calorimeter. Appl Thermal Eng. https://doi.org/10.1016/j.applthermaleng.2022.118428
Eshetu GG, Bertrand JP, Lecocq A, Grugeon S, Laruelle S, Armand M, Marlair G (2014) Fire behavior of carbonates-based electrolytes used in Li-ion rechargeable batteries with a focus on the role of the LiPF6 and LiFSI salts. J Power Sources 269:804–811. https://doi.org/10.1016/j.jpowsour.2014.07.065
Mei J, Liu H, Chen M (2020) Experimental study on combustion behavior of mixed carbonate solvents and separator used in lithium-ion batteries. J. Thermal Anal Calorim 139:1255–1264. https://doi.org/10.1007/s10973-019-08502-3
Fu Y, Lu S, Shi L, Cheng X, Zhang H (2016) Combustion characteristics of electrolyte pool fires for lithium ion batteries. J Electrochem Soc 163:A2022–A2028. https://doi.org/10.1149/2.0721609jes
Liu C, Huang Q, Zheng K, Qin J, Zhou D, Wang J (2020) Impact of lithium salts on the combustion characteristics of electrolyte under diverse pressures. Energies 13:5373. https://doi.org/10.3390/en13205373
Guo F, Ozaki Y, Nishimura K, Hashimoto N, Fujita O (2020) Influence of lithium salts on the combustion characteristics of dimethyl carbonate-based electrolytes using a wick combustion method. Combust Flame 213:314–321. https://doi.org/10.1016/j.combustflame.2019.12.001
Dahbi M, Ghamouss F, Tran-Van F, Lemordant D, Anouti M (2011) Comparative study of EC/DMC LiTFSI and LiPF6 electrolytes for electrochemical storage. J Power Sources 196:9743–9750. https://doi.org/10.1016/j.jpowsour.2011.07.071
Yang YP, Huang AC, Tang Y, Liu YC, Wu ZH, Zhou HL, Li ZP, Shu CM, Jiang JC, Xing ZX (2021) Thermal stability analysis of lithium-ion battery electrolytes based on lithium Bis(trifluoromethanesulfonyl)imide-lithium difluoro(oxalato)borate dual-salt. Polymers 13:707. https://doi.org/10.3390/polym13050707
Eshetu GG, Grugeon S, Laruelle S, Boyanov S, Lecocq A, Bertrand JP, Marlair G (2013) In-depth safety-focused analysis of solvents used in electrolytes for large scale lithium ion batteries. Phys Chem Chem Phys 15:9145. https://doi.org/10.1039/c3cp51315g
Mason DM, Gandhi KN (1983) Formulas for calculating the calorific value of coal and coal chars: development, tests, and uses. Fuel Process Technol 7:11–22
Ravdel B, Abraham KM, Gitzendanner R, Dicarlo J, Lucht B, Campion C (2003) Thermal stability of lithium-ion battery electrolytes. J Power Sources 119–121:805–810. https://doi.org/10.1016/s0378-7753(03)00257-x
Liu P, Liu C, Yang K, Zhang M, Gao F, Mao B, Li H, Duan Q, Wang Q (2020) Thermal runaway and fire behaviors of lithium iron phosphate battery induced by over heating. J Energy Storage 31:101714
Ghabache E, Seon T (2016) Size of the top jet drop produced by bubble bursting. Phys Rev Fluids. https://doi.org/10.1103/PhysRevFluids.1.051901
Zhang JQ, Chen JJJ, Zhou NJ (2012) Characteristics of jet droplet produced by bubble bursting on the free liquid surface. Chem Eng Sci 68:151–156. https://doi.org/10.1016/j.ces.2011.09.019
Zhang Z, He J, Yuen R and Wang J (2019) The effect of suspended carbon-black particles on the burning behavior of n-heptane pool fire. In: 12th Asia-Pacific Conference on Combustion, ASPACC 2019
Alibakhshi A, Mirshahvalad H, Alibakhshi S (2015) Investigating the mechanism of effect of carbon nanotubes on flame spread over liquid fuels. Fire Technol 51:759–770. https://doi.org/10.1007/s10694-014-0392-7
He YB, Li B, Liu M, Zhang C, Lv W, Yang C, Li J, Du H, Zhang B, Yang QH, Kim JK, Kang F (2012) Gassing in Li4Ti5O12-based batteries and its remedy. Sci Rep. https://doi.org/10.1038/srep00913
Janz GJ, Lorenz MR, Brown CT (1958) Preparation and thermal stability of lithium titanium fluoride. J Am Chem Soc 80:4126–4128. https://doi.org/10.1021/ja01549a003
Belharouak I, Koenig GM, Tan T, Yumoto H, Ota N, Amine K (2012) Performance degradation and gassing of Li4Ti5O12/limn2o4lithium-ion cells. J Electrochem Soc 159:A1165–A1170. https://doi.org/10.1149/2.013208jes
Acknowledgements
This work is supported by the National Key R&D Program of China (Grant No.2019YFA0705604). Q. S. Wang is supported by Youth Innovation Promotion Association CAS (Grant No. Y201768).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 180606 KB)
Supplementary file2 (MP4 186513 KB)
Supplementary file3 (MP4 190477 KB)
Supplementary file3 (MP4 236201 KB)
Supplementary file3 (MP4 155745 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Y., Cheng, S., Liu, P. et al. Experimental Study on Combustion Characteristics of Electrolytes and Slurries for Semi-Solid Lithium-ion Flow Battery. Fire Technol 59, 1199–1220 (2023). https://doi.org/10.1007/s10694-023-01384-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10694-023-01384-w