Abstract
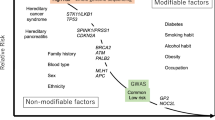
As in Western countries, familial pancreatic cancer accounts for 5–7% of pancreatic cancer (PC) in Japan. Opportunities for diagnosing hereditary pancreatic cancer (HPC) are increasing owing to the coverage of companion diagnostics and cancer genomic profiling by national health insurance in patients with unresectable or recurrent PC refractory to standard chemotherapies. HPC is recognized in 7% of PCs and 15% of familial pancreatic cancer, including germline variants of BRCA1/2, ATM, PALB2, APC, and mismatch repair genes. Individuals with 5-fold or greater inherited risks of PC are recommended to undergo pancreatic surveillance according to Japanese guidelines. The imaging modalities for this surveillance include endoscopic ultrasound, magnetic resonance cholangiopancreatography, abdominal ultrasound, and enhanced computed tomography. Currently, a nationwide prospective surveillance study is ongoing in Japan. Platinum-based chemotherapy is an effective pancreatic cancer treatment in patients with variants of homologous recombination repair genes (BRCA1/2 and PALB2); however, the use of platinum regimens solely based on familial/personal cancer history remains controversial. The efficacy of olaparib maintenance therapy, as confirmed by the POLO study, has significantly impacted the clinical treatment of advanced PC patients in Japan. Since the initiation of precision cancer medicine in 2019, genetic medicine for PC patients has expanded in Japan.


Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Okusaka T, Nakamura M, Yoshida M et al (2023) Clinical practice guidelines for pancreatic Cancer 2022 from the Japan Pancreas Society: a synopsis. Int J Clin Oncol 28:493–511
Kitano M, Morizane C, Hijioka S et al (2020) Surveillance for the early diagnosis of familial pancreatic cancer (Expert consensus). Suizo 35:322–330 (In Japanese with English abstract)
Matsubayashi H, Todaka A, Kawakami T et al (2023) Genetic medicine in companion diagnostics of germline BRCA testing of Japanese pancreatic cancer patients. J Hum Genet 68:81–86
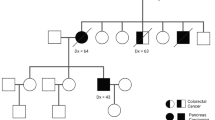
Hruban RH, Petersen GM, Goggins M et al (1999) Familial pancreatic cancer. Ann Oncol 10(Suppl 4):69–73
Wada K, Takaori K, Traverso LW et al (2013) Clinical importance of familial pancreatic Cancer Registry in Japan: a report from kick-off meeting at International Symposium on Pancreas Cancer 2012. J Hepatobiliary Pancreat Sci 20:557–566
Matsubayashi H, Takaori K, Morizane C et al (2017) Familial pancreatic cancer: Concept, management and issues. World J Gastroenterol 23:935–948
Matsubayashi H, Maeda A, Kanemoto H et al (2011) Risk factors of familial pancreatic cancer in Japan: current smoking and recent onset of diabetes. Pancreas 40:974–978
Takai E, Yachida S, Shimizu K et al (2016) Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget 7:74227–74235
Inoue M, Tajima K, Takezaki T et al (2003) Epidemiology of pancreatic cancer in Japan: a nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Int J Epidemiol 32:257–262
Mizukami K, Iwasaki Y, Kawakami E et al (2020) Genetic characterization of pancreatic cancer patients and prediction of carrier status of germline pathogenic variants in cancer-predisposing genes. EBioMedicine 60:103033
Takai E, Nakamura H, Chiku S et al (2022) Whole-exome sequencing reveals new potential susceptibility genes for Japanese familial pancreatic Cancer. Ann Surg 275:e652–e658
Roberts NJ, Norris AL, Petersen GM et al (2016) Whole genome sequencing defines the genetic heterogeneity of familial pancreatic Cancer. Cancer Discov 6:166–175
Goggins M, Overbeek KA, Brand R et al (2020) Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the pancreas Screening (CAPS) Consortium. Gut 69:7–17
Norris AL, Roberts NJ, Jones S et al (2015) Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam Cancer 14:95–103
Kessler MD, Damask A, O’Keeffe S et al (2022) Common and rare variant associations with clonal haematopoiesis phenotypes. Nature 612:301–309
Sunami K, Naito Y, Komine K et al (2022) Chronological improvement in precision oncology implementation in Japan. Cancer Sci 113:3995–4000
Higashigawa S, Matsubayashi H, Kiyozumi Y et al (2022) Present status of germline findings in precision medicine for Japanese cancer patients: issues in the current system. Jpn J Clin Oncol 52:599–608
Kondo T, Yamamoto Y, Fukuyama K et al (2022) Germline sequencing for presumed germline pathogenic variants via tumor-only comprehensive genomic profiling. Int J Clin Oncol 27:1256–1263
Japanese Organization of Hereditary Breast and Ovarian Cancer (JOHBOC) (2021) Guidelines for diagnosis and treatment of hereditary breast and ovarian cancer
Petrucelli N, Pal MBD T. BRCA1- and BRCA2-Associated Hereditary breast and ovarian Cancer. GeneReviews., 2022 (Revised).
Ueki A, Yoshida R, Kosaka T et al (2023) Clinical risk management of breast, ovarian, pancreatic, and prostatic cancers for BRCA1/2 variant carriers in Japan. J Hum Genet 68:517–526
Furukawa T, Sakamoto H, Takeuchi S et al (2015) Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep 5:8829
Tomita N, Ishida H, Tanakaya K et al (2021) Japanese Society for Cancer of the Colon and rectum (JSCCR) guidelines 2020 for the clinical practice of Hereditary Colorectal Cancer. Int J Clin Oncol 26:1353–1419
Matsubayashi H, Higashigawa S, Kiyozumi Y et al (2022) Microsatellite instability is biased in Amsterdam II-defined Lynch-related cancer cases with family history but is rare in other cancers: a summary of 1000 analyses. BMC Cancer 22:73
Akagi K, Oki E, Taniguchi H et al (2021) Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci 112:1105–1113
Dedeurwaerdere F, Claes KB, Van Dorpe J et al (2021) Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci Rep 11:12880
Loughrey MB, McGrath J, Coleman HG et al (2021) Identifying mismatch repair-deficient colon cancer: near-perfect concordance between immunohistochemistry and microsatellite instability testing in a large, population-based series. Histopathology 78:401–413
Matsubayashi H, Oishi T, Sasaki K et al (2023) Discordance of microsatellite instability and mismatch repair immunochemistry occurs depending on the cancer type. Hum Pathol 135:54–64
Hendifar AE, Larson BK, Rojansky R et al (2019) Pancreatic cancer ‘mismatch’ in Lynch syndrome. BMJ Open Gastroenterol 6:e000274
Takamizawa S, Morizane C, Tanabe N et al (2022) Clinical characteristics of pancreatic and biliary tract cancers associated with Lynch syndrome. J Hepatobiliary Pancreat Sci 29:377–384
Goggins M, Offerhaus GJ, Hilgers W et al (1998) Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am J Pathol 152:1501–1507
Tempero MA, Malafa MP, Benson AB, et al (2024) NCCN clinical practical guidelines in oncology. Pancreatic Adenocarcinoma ver.1. 2024 https://www.nccn.org/
Momozawa Y, Sasai R, Usui Y et al (2022) Expansion of Cancer Risk Profile for BRCA1 and BRCA2 pathogenic variants. JAMA Oncol 8:871–878
Vasen H, Ibrahim I, Ponce CG et al (2016) Benefit of Surveillance for Pancreatic Cancer in High-Risk individuals: outcome of long-term prospective Follow-Up studies from three European Expert centers. J Clin Oncol 34:2010–2019
Furniss CS, Yurgelun MB, Ukaegbu C et al (2021) Novel Models of Genetic Education and Testing for Pancreatic Cancer interception: preliminary results from the GENERATE Study. Cancer Prev Res (Phila) 14:1021–1032
Dbouk M, Katona BW, Brand RE et al (2022) The Multicenter Cancer of pancreas Screening Study: Impact on Stage and Survival. J Clin Oncol 40:3257–3266
Canto MI, Almario JA, Schulick RD et al (2018) Risk of neoplastic progression in individuals at high risk for pancreatic Cancer Undergoing Long-Term Surveillance. Gastroenterology 155:740–751e2
Klatte DCF, Boekestijn B, Onnekink AM et al (2023) Surveillance for pancreatic Cancer in high-risk individuals leads to Improved outcomes: a propensity score-matched analysis. Gastroenterology 164:1223–1231e4
Kanno A, Masamune A, Hanada K et al (2018) Multicenter study of early pancreatic cancer in Japan. Pancreatology 18:61–67
Hanada K, Shimizu A, Kurihara K et al (2022) Endoscopic approach in the diagnosis of high-grade pancreatic intraepithelial neoplasia. Dig Endosc 34:927–937
Ikezawa K, Tanaka S, Fukuda J et al (2023) Main pancreatic duct dilatation and pancreatic cysts in relatives and spouses of patients with pancreatic cancer. PLoS ONE 18:e0280403
Tanaka S, Nakao M, Ioka T et al (2010) Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: a prospective study. Radiology 254:965–972
Nakao M, Katayama K, Fukuda J et al (2017) Evaluating the ability to detect pancreatic lesions using a special ultrasonography examination focusing on the pancreas. Eur J Radiol 91:10–14
Hijioka S, Morizane C, Takaori K et al (2022) Study protocol for a multi-institutional prospective surveillance study among kindreds with familial pancreatic cancer and individuals with hereditary pancreatic cancer syndrome: the Diamond Study. Pancreatology 22:534–538
Okano N, Morizane C, Nomura S et al (2020) Phase II clinical trial of gemcitabine plus oxaliplatin in patients with metastatic pancreatic adenocarcinoma with a family history of pancreatic/breast/ovarian/prostate cancer or personal history of breast/ovarian/prostate cancer (FABRIC study). Int J Clin Oncol 25:1835–1843
Emelyanova M, Pudova E, Khomich D et al (2022) Platinum-based chemotherapy for pancreatic cancer: impact of mutations in the homologous recombination repair and fanconi anemia genes. Ther Adv Med Oncol 14:17588359221083050
Wattenberg MM, Asch D, Yu S et al (2020) Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer 122:333–339
Reiss KA, Yu S, Judy R et al (2018) Retrospective survival analysis of patients with Advanced pancreatic ductal adenocarcinoma and germline BRCA or PALB2 mutations. JCO Precis Oncol 2:1–9
Kitamura H, Morizane C, Tanabe N et al (2023) Clinical features of germline BRCA1/2 or ATM pathogenic variant positive pancreatic cancer in Japan. Pancreatology
Reiss KA, Mick R, O’Hara MH et al (2021) Phase II study of maintenance rucaparib in patients with platinum-sensitive Advanced Pancreatic Cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol 39:2497–2505
Gruber JJ, Afghahi A, Timms K et al (2022) A phase II study of talazoparib monotherapy in patients with wild-type BRCA1 and BRCA2 with a mutation in other homologous recombination genes. Nat Cancer 3:1181–1191
Schram AM, Colombo N, Arrowsmith E et al (2023) Avelumab Plus Talazoparib in patients with BRCA1/2- or ATM-Altered Advanced Solid Tumors: results from JAVELIN BRCA/ATM, an Open-Label, Multicenter, phase 2b, tumor-agnostic trial. JAMA Oncol 9:29–39
Golan T, Hammel P, Reni M et al (2019) Maintenance olaparib for germline BRCA-Mutated metastatic pancreatic Cancer. N Engl J Med 381:317–327
Marabelle A, Le DT, Ascierto PA et al (2020) Efficacy of Pembrolizumab in patients with Noncolorectal high microsatellite Instability/Mismatch repair-deficient Cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1–10
Latham A, Srinivasan P, Kemel Y et al (2019) Microsatellite instability is Associated with the Presence of Lynch Syndrome Pan-cancer. J Clin Oncol 37:286–295
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Both H.M and C.M wrote the main manuscript and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsubayashi, H., Morizane, C. Familial and hereditary pancreatic cancer in Japan. Familial Cancer (2024). https://doi.org/10.1007/s10689-024-00395-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10689-024-00395-y