Abstract
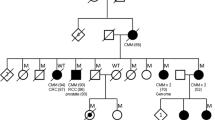
While several high-penetrance melanoma risk genes are known, variation in these genes fail to explain melanoma susceptibility in a large proportion of high-risk families. As part of a melanoma family sequencing study, including 435 families from Mediterranean populations we identified a novel NRAS variant (c.170A > C, p.D57A) in an Italian melanoma-prone family. This variant is absent in exomes in gnomAD, ESP, UKBiobank, and the 1000 Genomes Project, as well as in 11,273 Mediterranean individuals and 109 melanoma-prone families from the US and Australia. This variant occurs in the GTP-binding pocket of NRAS. Differently from other RAS activating alterations, NRAS D57A expression is unable to activate MAPK-pathway both constitutively and after stimulation but enhances EGF-induced PI3K-pathway signaling in serum starved conditions in vitro. Consistent with in vitro data demonstrating that NRAS D57A does not enrich GTP binding, molecular modeling suggests that the D57A substitution would be expected to impair Mg2 + binding and decrease nucleotide-binding and GTPase activity of NRAS. While we cannot firmly establish NRAS c.170A > C (p.D57A) as a melanoma susceptibility variant, further investigation of NRAS as a familial melanoma gene is warranted.




Similar content being viewed by others
Data availability
The data that support the findings will be available in dbGaP (https://www.ncbi.nlm.nih.gov/gap/) following a 6 month embargo from the date of publication.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Florell SR, Boucher KM, Garibotti G et al (2005) Population-based analysis of prognostic factors and survival in familial melanoma. J Clin Oncol 23(28):7168–7177. https://doi.org/10.1200/JCO.2005.11.999
Glazer AM, Winkelmann RR, Farberg AS, Rigel DS (2017) Analysis of Trends in US Melanoma Incidence and Mortality. JAMA Dermatol 153(2):225–226. https://doi.org/10.1001/jamadermatol.2016.4512
Whiteman DC, Green AC, Olsen CM (2016) The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol 136(6):1161–1171. https://doi.org/10.1016/j.jid.2016.01.035
Potrony M, Badenas C, Aguilera P et al (2015) Update in genetic susceptibility in melanoma. Ann Transl Med 3(15):210. https://doi.org/10.3978/j.issn.2305-5839.2015.08.11
Colombino M, Capone M, Lissia A et al (2012) BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol 30(20):2522–2529. https://doi.org/10.1200/JCO.2011.41.2452
Cirstea IC, Kutsche K, Dvorsky R et al (2010) A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat Genet 42(1):27–29. https://doi.org/10.1038/ng.497
Shi J, Yang XR, Ballew B et al (2014) Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet 46(5):482–486. https://doi.org/10.1038/ng.2941
Gelb BD, Cave H, Dillon MW et al (2018) ClinGen’s RASopathy Expert Panel consensus methods for variant interpretation. Genet Med 20(11):1334–1345. https://doi.org/10.1038/gim.2018.3
Burd CE, Liu W, Huynh MV et al (2014) Mutation-specific RAS oncogenicity explains NRAS codon 61 selection in melanoma. Cancer Discov 4(12):1418–1429. https://doi.org/10.1158/2159-8290.CD-14-0729
Jakob JA, Bassett RL Jr, Ng CS et al (2012) NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 118(16):4014–4023. https://doi.org/10.1002/cncr.26724
Martinelli S, Stellacci E, Pannone L et al (2015) Molecular diversity and associated phenotypic spectrum of germline CBL mutations. Hum Mutat 36(8):787–796. https://doi.org/10.1002/humu.22809
Landi MT, Bishop DT, MacGregor S et al (2020) Genome-wide association meta-analyses combining multiple risk phenotypes provide insights into the genetic architecture of cutaneous melanoma susceptibility. Nat Genet 52(5):494–504. https://doi.org/10.1038/s41588-020-0611-8
Stratigos AJ, Fargnoli MC, De Nicolo A et al (2018) MelaNostrum: a consensus questionnaire of standardized epidemiologic and clinical variables for melanoma risk assessment by the melanostrum consortium. J Eur Acad Dermatol Venereol 32(12):2134–2141. https://doi.org/10.1111/jdv.15208
Landi MT, Consonni D, Rotunno M et al (2008) Environment and genetics in lung cancer etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC Public Health 8:203. https://doi.org/10.1186/1471-2458-8-203
Knihtila R, Holzapfel G, Weiss K, Meilleur F, Mattos C (2015) Neutron crystal structure of RAS gtpase puts in question the protonation state of the GTP gamma-phosphate. J Biol Chem 290(52):31025–31036. https://doi.org/10.1074/jbc.M115.679860
Shirouzu M, Koide H, Fujita-Yoshigaki J et al (1994) Mutations that abolish the ability of Ha-Ras to associate with Raf-1. Oncogene 9(8):2153–2157
Nussinov R, Tsai CJ, Muratcioglu S, Jang H, Gursoy A, Keskin O (2015) Principles of K-Ras effector organization and the role of oncogenic K-Ras in cancer initiation through G1 cell cycle deregulation. Expert Rev Proteomics 12(6):669–682. https://doi.org/10.1586/14789450.2015.1100079
Nussinov R, Zhang M, Tsai CJ, Liao TJ, Fushman D, Jang H (2018) Autoinhibition in Ras effectors Raf, PI3Kalpha, and RASSF5: a comprehensive review underscoring the challenges in pharmacological intervention. Biophys Rev 10(5):1263–1282. https://doi.org/10.1007/s12551-018-0461-0
Zhang M, Jang H, Nussinov R (2019) The structural basis for Ras activation of PI3Kalpha lipid kinase. Phys Chem Chem Phys 21(22):12021–12028. https://doi.org/10.1039/c9cp00101h
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164. https://doi.org/10.1093/nar/gkq603
Li Q, Wang K (2017) InterVar: Clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am J Hum Genet 100(2):267–280. https://doi.org/10.1016/j.ajhg.2017.01.004
Robles-Espinoza CD, Harland M, Ramsay AJ et al (2014) POT1 loss-of-function variants predispose to familial melanoma. Nat Genet 46(5):478–481. https://doi.org/10.1038/ng.2947
Jang H, Muratcioglu S, Gursoy A, Keskin O, Nussinov R (2016) Membrane-associated Ras dimers are isoform-specific: K-Ras dimers differ from H-Ras dimers. Biochem J 473(12):1719–1732. https://doi.org/10.1042/BCJ20160031
Brooks BR, Brooks CL 3rd, Mackerell AD Jr et al (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30(10):1545–1614. https://doi.org/10.1002/jcc.21287
Phillips JC, Braun R, Wang W et al (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26(16):1781–1802. https://doi.org/10.1002/jcc.20289
Acknowledgements
All simulations were performed using the high-performance computational facilities of the Biowulf PC/Linux cluster at the National Institutes of Health, Bethesda, MD (https://hpc.nih.gov/). Membership of the Melanostrum Consortium can be found at the following link: https://dceg.cancer.gov/research/cancer-types/melanoma/melanostrum
Funding
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics (ZIACP010201 for KMB, ZIACP101231 for MTL, and ZIACP010144 for MS; https://dceg.cancer.gov/) and Center for Cancer Research (ZIABC010442 for RN; https://ccr.cancer.gov/) and the National Health and Medical Research Council of Australia (1117663 for NKH; https://www.nhmrc.gov.au). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: MTL, KB, DS, JK, MT, MS, TA, NH, TZ, RN; Data Curation: BZ, TZ; Formal Analysis: BZ, TZ, RN, MZ, HJ, MX; Funding Acquisition: MTL, KB, RN; Investigation: MX, KJ, BH; Methodology: MX; Project Administration: DC, AP, LM; Resources: MCF, KP, AS, CM, PG, SP, EN, DC; Supervision: MTL, KB; V: AG, XRY, NH; Visualization: MX, KB, MZ; Writing—Original Draft Preparation: KB, MTL, MX, MS, RN, MZ; Writing—Review and Editing: DS, NH, CM, MCF, PG, AG, EN, KB, MTL.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the National Cancer Institute (Protocol: 02CN038).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Membership of the MelaNostrum Consortium can be found here: https://dceg.cancer.gov/research/cancer-types/melanoma/melanostrum
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brown, K.M., Xu, M., Sargen, M. et al. Novel MAPK/AKT-impairing germline NRAS variant identified in a melanoma-prone family. Familial Cancer 21, 347–355 (2022). https://doi.org/10.1007/s10689-021-00267-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-021-00267-9