Abstract
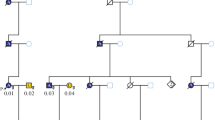
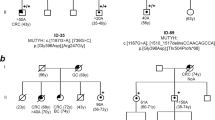
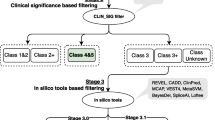
In up to 30 % of patients with colorectal adenomatous polyposis, no germline mutation in the known genes APC, causing familial adenomatous polyposis, MUTYH, causing MUTYH-associated polyposis, and POLE or POLD1, causing Polymerase-Proofreading-associated polyposis can be identified, although a hereditary etiology is likely. To uncover new causative genes, exome sequencing was performed using DNA from leukocytes and a total of 12 colorectal adenomas from seven unrelated patients with unexplained sporadic adenomatous polyposis. For data analysis and variant filtering, an established bioinformatics pipeline including in-house tools was applied. Variants were filtered for rare truncating point mutations and copy-number variants assuming a dominant, recessive, or tumor suppressor model of inheritance. Subsequently, targeted sequence analysis of the most promising candidate genes was performed in a validation cohort of 191 unrelated patients. All relevant variants were validated by Sanger sequencing. The analysis of exome sequencing data resulted in the identification of rare loss-of-function germline mutations in three promising candidate genes (DSC2, PIEZO1, ZSWIM7). In the validation cohort, further variants predicted to be pathogenic were identified in DSC2 and PIEZO1. According to the somatic mutation spectra, the adenomas in this patient cohort follow the classical pathways of colorectal tumorigenesis. The present study identified three candidate genes which might represent rare causes for a predisposition to colorectal adenoma formation. Especially PIEZO1 (FAM38A) and ZSWIM7 (SWS1) warrant further exploration. To evaluate the clinical relevance of these genes, investigation of larger patient cohorts and functional studies are required.

Similar content being viewed by others
References
Galiatsatos P, Foulkes WD (2006) Familial adenomatous polyposis. Am J Gastroenterol 101(2):385–398
Aretz S, Stienen D, Friedrichs N, Stemmler S, Uhlhaas S, Rahner N, Propping P, Friedl W (2007) Somatic APC mosaicism: a frequent cause of familial adenomatous polyposis (FAP). Hum Mutat 28(10):985–992
Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker E, Vasen HF, Breuning MH, Tops CM (2008) Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut 57(1):71–76
Spier I, Drichel D, Kerick M, Kirfel J, Horpaopan S, Laner A, Holzapfel S, Peters S, Adam R, Zhao B, Becker T, Lifton RP, Perner S, Hoffmann P, Kristiansen G, Timmermann B, Nothen MM, Holinski-Feder E, Schweiger MR, Aretz S (2015) Low-level APC mutational mosaicism is the underlying cause in a substantial fraction of unexplained colorectal adenomatous polyposis cases. J Med Genet. doi:10.1136/jmedgenet-2015-103468
Spier I, Horpaopan S, Vogt S, Uhlhaas S, Morak M, Stienen D, Draaken M, Ludwig M, Holinski-Feder E, Nothen MM, Hoffmann P, Aretz S (2012) Deep intronic APC mutations explain a substantial proportion of patients with familial or early-onset adenomatous polyposis. Hum Mutat 33(7):1045–1050
Mazzei F, Viel A, Bignami M (2013) Role of MUTYH in human cancer. Mutat Res 743–744:33–43
Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I, Sherborne A, Chubb D, Carvajal-Carmona LG, Ma Y, Kaur K, Dobbins S, Barclay E, Gorman M, Martin L, Kovac MB, Humphray S, Lucassen A, Holmes CC, Bentley D, Donnelly P, Taylor J, Petridis C, Roylance R, Sawyer EJ, Kerr DJ, Clark S, Grimes J, Kearsey SE, Thomas HJ, McVean G, Houlston RS, Tomlinson I (2013) Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 45(2):136–144
Spier I, Holzapfel S, Altmuller J, Zhao B, Horpaopan S, Vogt S, Chen S, Morak M, Raeder S, Kayser K, Stienen D, Adam R, Nurnberg P, Plotz G, Holinski-Feder E, Lifton RP, Thiele H, Hoffmann P, Steinke V, Aretz S (2015) Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int J Cancer 137(2):320–331
Weren RDA, Ligtenberg MJL, Kets CM, de Voer RM, Verwiel ETP, Spruijt L, van Zelst-Stams WAG, Jongmans MC, Gilissen C, Hehir-Kwa JY, Hoischen A, Shendure J, Boyle EA, Kamping EJ, Nagtegaal ID, Tops BBJ, Nagengast FM, Geurts van Kessel A, van Krieken JHJM, Kuiper RP, Hoogerbrugge N (2015) A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet 47(6):668–671
Gilissen C, Hoischen A, Brunner HG, Veltman JA (2012) Disease gene identification strategies for exome sequencing. Eur J Hum Genet 20(5):490–497
Aretz S, Stienen D, Uhlhaas S, Pagenstecher C, Mangold E, Caspari R, Propping P, Friedl W (2005) Large submicroscopic genomic APC deletions are a common cause of typical familial adenomatous polyposis. J Med Genet 42(2):185–192
Horpaopan S, Spier I, Zink AM, Altmuller J, Holzapfel S, Laner A, Vogt S, Uhlhaas S, Heilmann S, Stienen D, Pasternack SM, Keppler K, Adam R, Kayser K, Moebus S, Draaken M, Degenhardt F, Engels H, Hofmann A, Nothen MM, Steinke V, Perez-Bouza A, Herms S, Holinski-Feder E, Frohlich H, Thiele H, Hoffmann P, Aretz S (2015) Genome-wide CNV analysis in 221 unrelated patients and targeted high-throughput sequencing reveal novel causative candidate genes for colorectal adenomatous polyposis. Int J Cancer 136(6):E578–589
Schweiger MR, Kerick M, Timmermann B, Albrecht MW, Borodina T, Parkhomchuk D, Zatloukal K, Lehrach H (2009) Genome-wide massively parallel sequencing of formaldehyde fixed-paraffin embedded (FFPE) tumor tissues for copy-number- and mutation-analysis. PLoS ONE 4(5):e5548
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25(14):1754–1760
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9):1297–1303
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164
Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB (2013) Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet 9(8):e1003709
Huang N, Lee I, Marcotte EM, Hurles ME (2010) Characterising and predicting haploinsufficiency in the human genome. PLoS Genet 6(10):e1001154
Voorham QJ, Carvalho B, Spiertz AJ, Claes B, Mongera S, van Grieken NC, Grabsch H, Kliment M, Rembacken B, van de Wiel MA, Quirke P, Mulder CJ, Lambrechts D, van Engeland M, Meijer GA (2012) Comprehensive mutation analysis in colorectal flat adenomas. PLoS ONE 7(7):e41963
Funakoshi S, Ezaki T, Kong J, Guo RJ, Lynch JP (2008) Repression of the desmocollin 2 gene expression in human colon cancer cells is relieved by the homeodomain transcription factors Cdx1 and Cdx2. Mol Cancer Res 6(9):1478–1490
Knosel T, Chen Y, Hotovy S, Settmacher U, Altendorf-Hofmann A, Petersen I (2012) Loss of desmocollin 1-3 and homeobox genes PITX1 and CDX2 are associated with tumor progression and survival in colorectal carcinoma. Int J Colorectal Dis 27(11):1391–1399
Kolegraff K, Nava P, Helms MN, Parkos CA, Nusrat A (2011) Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/beta-catenin signaling. Mol Biol Cell 22(8):1121–1134
Martin V, Chahwan C, Gao H, Blais V, Wohlschlegel J, Yates JR 3rd, McGowan CH, Russell P (2006) Sws1 is a conserved regulator of homologous recombination in eukaryotic cells. EMBO J 25(11):2564–2574
McHugh BJ, Murdoch A, Haslett C, Sethi T (2012) Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS ONE 7(7):e40346
Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J (2012) Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484(7395):546–549
Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad KR, Evans PC, Ainscough JF, Beech DJ (2014) Piezo1 integration of vascular architecture with physiological force. Nature 515(7526):279–282
Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garcon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A (2013) Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun 4:1884
MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C (2014) Guidelines for investigating causality of sequence variants in human disease. Nature 508(7497):469–476
Acknowledgments
We thank the patients and their families for participating in the study. We are grateful to Susanne Raeder, Dietlinde Stienen, and Siegfried Uhlhaas for their excellent technical support.
Funding
This work was supported by the German Cancer Aid (Deutsche Krebshilfe e.V. Bonn, Grant number 108421); the Gerok-Stipendium of the University Hospital Bonn (Grant no. O-149.0098); the NIH Centers for Mendelian Genomics (5U54HG006504); the Federal Ministry of Education and Research (0316190A); and the Volkswagenstiftung (Lichtenberg Program to M.R.S.). These funding sources had no involvement in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the manuscript for publication. The corresponding author had full access to all study data, and had final responsibility for the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Spier, I., Kerick, M., Drichel, D. et al. Exome sequencing identifies potential novel candidate genes in patients with unexplained colorectal adenomatous polyposis. Familial Cancer 15, 281–288 (2016). https://doi.org/10.1007/s10689-016-9870-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-016-9870-z