Abstract
Desmoid tumors are locally invasive myofibroblastic lesions that arise predominantly in the abdominal wall or shoulder girdle and are prone to aggressive local recurrences without metastases. We hypothesized the intrinsic invasiveness and drug resistance displayed by cells derived from a familial adenomatous polyposis (FAP)-associated desmoid tumor would surpass the response shown by cells derived from sporadic desmoid tumors. In vitro cell motility and expression of motility-associated genes were quantified using Boyden Chambers and Enzyme-Linked ImmunoSorbent Assays, respectively. Doxorubicin resistance was quantified by Trypan Blue dye exclusion. cDNA microarrays identified genes responsive to doxorubicin. FAP-associated tumor cells were significantly more invasive and refractory to doxorubicin than were cells extracted from sporadic tumors. Pro-MMP1 protein predominated over MMP3 in FAP-associated cell culture supernatants, while MMP3 was the dominant antigen in sporadic tumor cell supernatants. Three genes associated with apoptosis were identified by microarray, two prosurvival genes overexpressed in FAP-associated cell cultures (NTN1, TNFRSF10C) and one proapoptosis gene overexpressed in sporadic tumor cell cultures (FOXL2).





Similar content being viewed by others
Abbreviations
- FAP:
-
Familial adenomatous polyposis
- APC:
-
Adenomatous polyposis coli
- EGFR:
-
Epidermal growth factor receptor
- rhEGF:
-
Recombinant human epidermal growth factor
References
Alman BA, Pajerski ME, Diaz-Cano S et al (1997) Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol 6:98–101
Angiero F, Benedicenti S, Stefani M (2008) Fibromatosis of the head and neck: morphological, immunohistochemical and clinical features. Anticancer Res 28:1725–1732
Biermann JS (2000) Desmoid tumors. Curr Treat Options Oncol 1:262–266
Burke AP, Sobin LH, Shekitka KM (1990) Mesenteric fibromatosis. A follow-up study. Arch Pathol Lab Med 114:832–835
Cheng S, Tada M, Hida Y et al (2008) High MMP-1 mRNA expression is a risk factor for disease-free and overall survivals in patients with invasive breast carcinoma. J Surg Res 146:104–109
Cohen S, Ad-El D, Benjaminov O et al (2008) Post-traumatic soft tissue tumors: case report and review of the literature a propos a post-traumatic paraspinal desmoid tumor. World J Surg Oncol 6:28
Debatin KM (1999) Activation of apoptosis pathways by anticancer drugs. Adv Exp Med Biol 457:237–244
De Cian F, Delay E, Rudigoz RC et al (1999) Desmoid tumor arising in a cesarean section scar during pregnancy: monitoring and management. Gynecol Oncol 75:145–148
Denys H, De Wever O, Nusgens B et al (2004) Invasion and MMP expression profile in desmoid tumours. Br J Cancer 90:1443–1449
Dimanche-Boitrel MT, Genne P, Duchamp O, Chauffert B (1994) Confluence dependent resistance (CDR) to doxorubicin and E-cadherin expression in murine mammary cells. Cancer Lett 85:171–176
Dobbin K, Shih JH, Simon R (2003) Statistical design of reverse dye microarrays. Bioinformatics 19:803–810
Ezumi K, Yamamoto H, Takemasa I et al (2008) Dacarbazine–Doxorubicin therapy ameliorated an extremely aggressive mesenteric desmoid tumor associated with familial adenomatous polyposis: report of a case. Jpn J Clin Oncol 38:222–226
Fallen T, Wilson M, Morlan B et al (2006) Desmoid tumors—a characterization of patients seen at Mayo Clinic 1976–1999. Fam Cancer 5:191–194
Fang Y, Sullivan R, Graham CH (2007) Confluence-dependent resistance to doxorubicin in human MDA-MB-231 breast carcinoma cells requires hypoxia-inducible factor-1 activity. Exp Cell Res 313:866–877
Ferenc T, Sygut J, Kopczyński J et al (2006) Aggressive fibromatosis (desmoid tumors): definition, occurrence, pathology, diagnostic problems, clinical behavior, genetic background. Pol J Pathol 57:5–15
Fitamant J, Guenebeaud C, Coissieux MM et al (2008) Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA 105:4850–4855
Gouazé V, Mirault ME, Carpentier S, Salvayre R, Levade T, Andrieu-Abadie N (2001) Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol Pharmacol 60:488–496
Hosalkar HS, Torbert JT, Fox EJ et al (2008) Musculoskeletal desmoid tumors. J Am Acad Orthop Surg 16:188–198
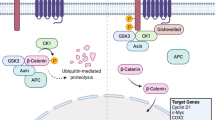
Jilong Y, Jian W, Xiaoyan Z et al (2007) Analysis of APC/beta-catenin genes mutations and Wnt signalling pathway in desmoid-type fibromatosis. Pathology 39:319–325
Joyner DE, Albritton KH, Bastar JD et al (2006) G3139 antisense oligonucleotide directed against antiapoptotic Bcl-2 enhances doxorubicin cytotoxicity in the FU-SY-1 synovial sarcoma cell line. J Orthop Res 24:474–480
Knudsen AL, Bülow S (2001) Desmoid tumour in familial adenomatous polyposis. A review of literature. Fam Cancer 1:111–119
Kong Y, Poon R, Nadesan P et al (2004) Matrix metalloproteinase activity modulates tumor size, cell motility, and cell invasiveness in murine aggressive fibromatosis. Cancer Res 64:5795–5803
Kotiligam D, Lazar AJ, Pollock RE et al (2008) Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol 23:117–126
Kudoh K, Ramanna M, Ravatn R et al (2000) Monitoring the expression profiles of doxorubicin induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res 60:4161–4166
Lee K, Pisarska MD, Ko JJ et al (2005) Transcriptional factor FOXL2 interacts with DP103 and induces apoptosis. Biochem Biophys Res Commun 336:876–881
Leithner A, Gapp M, Leithner K et al (2004) Margins in extra-abdominal desmoid tumors: a comparative analysis. J Surg Oncol 86:152–156
Lund-Johansen M, Bjerkvig R, Humphrey PA et al (1990) Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res 50:6039–6044
Mace J, Sybil Biermann J, Sondak V, McGinn C, Hayes C, Thomas D, Baker L (2002) Response of extraabdominal desmoid tumors to therapy with imatinib mesylate. Cancer 95:2373–2379
Messersmith WA, Ahnen DJ (2008) Targeting EGFR in colorectal cancer. N Engl J Med 359:1834–1836
Mills BG, Frausto A, Brien E (2000) Cytokines associated with the pathophysiology of aggressive fibromatosis. J Orthop Res 18:655–662
Momparler RL, Karon M, Siegel SE et al (1976) Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res 36:2891–2895
Muller C, Laurent G, Ling V (1995) P-glycoprotein stability is affected by serum deprivation and high cell density in multidrug-resistant cells. J Cell Physiol 163:538–544
Okuno S (2006) The enigma of desmoid tumors. Curr Treat Options Oncol 7:438–443
Pryczynicz A, Guzińska-Ustymowicz K, Kemona A et al (2008) Expression of EGF and EGFR strongly correlates with metastasis of pancreatic ductal carcinoma. Anticancer Res 28:1399–1404
Pulukuri SM, Rao JS (2008) Matrix metalloproteinase-1 promotes prostate tumor growth and metastasis. Int J Oncol 32:757–765
Rosenzweig BA, Pine PS, Domon OE et al (2004) Dye bias correction in dual-labeled cDNA microarray gene expression measurements. Environ Health Perspect 112:480–487
Saito T, Oda Y, Kawaguchi K et al (2002) Possible association between higher beta-catenin mRNA expression and mutated beta-catenin in sporadic desmoid tumors: real-time semiquantitative assay by TaqMan polymerase chain reaction. Lab Invest 82:97–103
Sturt NJ, Gallagher MC, Bassett P, Philp CR, Neale KF, Tomlinson IP, Silver AR, Phillips RK (2004) Evidence for genetic predisposition to desmoid tumours in familial adenomatous polyposis independent of the germline APC mutation. Gut 53:1832–1836
Sturt NJ, Clark SK (2006) Current ideas in desmoid tumours. Fam Cancer 5:275–285
Tejpar S, Michils G, Denys H et al (2005) Analysis of Wnt/Beta catenin signalling in desmoid tumors. Acta Gastroenterol Belg 68:5–9
Tejpar S, Nollet F, Li C et al (1999) Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 18:6615–6620
Tolan S, Shanks JH, Loh MY et al (2007) Fibromatosis: benign by name but not necessarily by nature. Clin Oncol (R Coll Radiol) 19:319–326
Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B (2004) Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem 279:25535–25543
Wang YD, Yang F, Chen WD, Huang X, Lai L, Forman BM, Huang W (2008) Farnesoid X receptor protects liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo. Mol Endocrinol 22:1622–1632
Zhang XD, Nguyen T, Thomas WD et al (2000) Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett 482:193–199
Acknowledgments
This study was supported by research grants from the Desmoid Tumor Research Foundation (DTRF) and the Huntsman Cancer Foundation. The authors thank Diane M. Miller for assistance with the figures and acknowledge the support provided by the Microarray Core Facility, Huntsman Cancer Institute, and the NCI Cancer Center support grant P30CA042014.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10689-009-9304-2
Rights and permissions
About this article
Cite this article
Joyner, D.E., Trang, S.H., Aboulafia, A.J. et al. FAP-associated desmoid invasiveness correlates with in vitro resistance to doxorubicin. Familial Cancer 8, 569–580 (2009). https://doi.org/10.1007/s10689-009-9288-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-009-9288-y