Abstract
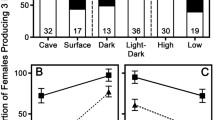
Obligate and facultative cave species both play significant functional roles in cave ecosystems. Unlike obligate cave species, facultative cave species can persist in habitats both within and outside of caves. However, no comparative demographic model explaining the sustained presence of both obligate and facultative cave species has been provided. We developed a multi-state capture–mark–recapture (CMR) analysis based on 5 years of data collected from caves in northern Alabama, USA to explore differences in survival and reproductive transition probabilities between obligate (Orconectes australis and Cambarus hamulatus) and facultative (C. tenebrosus) cave crayfish. Multi-state CMR analyses revealed that male obligate cave species showed significantly higher rates of transitioning to a reproductive state than male C. tenebrosus, while no differences among species were observed for females. Transitioning into a non-reproductive state, however, was higher for obligate cave species regardless of sex. Apparent survival rates between cave obligates and C. tenebrosus did not differ, suggesting that the larger population sizes of obligate cave species within our study sites may be driven by more successful male reproductive strategies. Our results suggest that obligate cave crayfishes have evolved unique sex-specific reproductive strategies not shared by C. tenebrosus that likely represent a specialized adaptation to the cave environment. Conversely, persistent immigration by surface populations is likely crucial for the sustained presence of facultative species within cave environments.




Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Aiken D (1969) Ovarian maturation and egg laying in the crayfish Orconectes virilis: influence of temperature and photoperiod. Can J Zool 47:931–935
Ameyaw-Akumfi C (1981) Courtship in the crayfish Procambarus clarkii (Girard) (Decapoda, Astacidea). Crustaceana 40:57–64
Aquiloni L, Gherardi F (2008) Extended mother-offspring relationships in crayfish: the return behavior of juvenile Procambarus clarkia. Ethology 114:946–954
Boretto J, Cabezas F, Tappari F et al (2014) Reproductive biology of Phymaturus spectabilis (Liolaemidae): females skip reproduction in cold and harsh environments of Patagonia, Argentina. Herpetol Conserv Biol 9:170–180
Brewis J, Bowler K (1985) A study of reproductive females of the freshwater crayfish Austropotamobius pallipes. Hydrobiologia 121:145–149
Bronikowski A (2000) Experimental evidence for the adaptive evolution of growth rate in the garter snake Thamnophis elegans. Evolution 54:1760–1767
Bronikowski A, Arnold S (1999) The evolutionary ecology of life history variation in the garter snake Thamnophis elegans. Ecology 80:2314–2325
Burnham K, Anderson D (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Caswell H (2001) Matrix population models: construction, analysis, and interpretation, 2nd edn. Sinauer Associates Inc, Sunderland
Cayuela H, Besnard A, Bonnaire E et al (2014) To breed or not to breed: past reproductive status and environmental cues drive current breeding decisions in a long-lived amphibian. Oecologia 176:107–116
Chevin L, Hoffmann A (2017) Evolution of phenotypic plasticity in extreme environments. Philos Trans R Soc B: Biol Sci 372:20160138.https://dx.doi.org/10.1098/rstb.2016.0138
Choquet R, Lebreton J, Gimenez O et al (2009a) U-CARE: Utilities for performing goodness of fit tests and manipulating capture–recapture data. Ecography 32:1071–1074
Choquet R, Rouan L, Pradel R (2009b) Program E-SURGE: a software application for fitting multievent models. In: Thomson DL, Cooch EG, Conroy MJ (eds) Modeling demographic processes in marked populations. Springer, New York, pp 845–865
Coulson T, Catchpole E, Albon S et al (2001) Age, sex, density, winter weather, and population crashes in Soay sheep. Science 292:1528–1531
Covich A, Thorp J (1991) Crustacea: Introduction and Peracarida. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, pp 665–689
Culver D (1985) Trophic relationships in aquatic cave environments. Stygologia 1:43–53
Engel A (2007) Observations on the biodiversity of sulfidic karst habitats. J Cave Karst Stud 69:187–206
Engen S, Sæther B (2017) r- and K-selection in fluctuating populations is determined by the evolutionary trade-off between two fitness measures: growth rate and lifetime reproductive success. Evolution 71:167–173
Fagan W, Cosner C, Larsen E et al (2010) Reproductive asynchrony in spatial population models: how mating behavior can modulate Allee effects arising from isolation in both space and time. Am Nat 175:362–373
Gerber L, White E (2014) Two-sex matrix models in assessing population viability: when do male dynamics matter? J Appl Ecol 51:270–278
Gimenez O, Lebreton J, Choquet R et al (2018) R2ucare: An R package to perform goodness-of-fit tests for capture–recapture models. Methods Ecol Evol 9:1749–1754
Gutiérrez-Yurrita P, Montes C (1999) Bioenergetics and phenology of reproduction of the introduced red swamp crayfish, Procambarus clarkii, in Doñana National Park, Spain, and implications for species management. Freshw Biol 42:561–574
Hayward A, Gillooly J (2011) The cost of sex: Quantifying energetic investment in gamete production by males and females. PLoS ONE 6:e16557
Henderson BA, Trivedi T, Collins N (2000) Annual cycle of energy allocation to growth and reproduction of yellow perch. J Fish Biol 57:122–133
Hervant F, Mathieu J, Durand J (2001) Behavioural, physiological and metabolic responses to long-term starvation and refeeding in a blind cave-dwelling (Proteus anguinus) and a surface-dwelling (Euproctus asper) salamander. J Exp Biol 204:269–281
Hunter J, Macewicz B, Sibert J (1986) The spawning frequency of skipjack tuna, Katsuwonus pelamis, from the South Pacific. Fish Bull 84:895–903
Huntsman B, Venarsky M, Benstead J et al (2011a) Effects of organic matter availability on the life history and production of a top vertebrate predator (Plethodontidae: Gyrinophilus palleucus) in two cave streams. Freshw Biol 56:1746–1760
Huntsman B, Venarsky M, Benstead J (2011b) Relating carrion breakdown rates to ambient resource level and community structure in four cave stream ecosystems. J N Am Benthol Soc 30:882–892
Hüppop K (2000) How do cave animals cope with the food scarcity in caves? In: Goodall DW, Wilkens H, Culver DC, Humphreys WF (eds) Ecosystems of the world: subterranean ecosystems. Elsevier Science, Amsterdam, pp 159–188
Jegla T (1966) Reproductive and molting cycles in cave crayfish. Biol Bull 130:345–358
Jenouvrier S, Barbraud C, Cazelles B et al (2005) Modelling population dynamics of seabirds: importance of the effects of climate fluctuations on breeding proportions. Oikos 108:511–522
Jurcak A, Lahman S, Wofford S, Moore P (2016) Behavior of crayfish. In: Longshaw M, Stebbing B (eds) Biology and ecology of crayfish. CRC Press, London, pp 117–131
Kotliar B, Wiens J (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59:253–260
Lebreton J, Nichols J, Barker R et al (2009) Modeling individual animal histories with multistate capture–recapture models. Adv Ecol Res 41:87–173
Leibold M, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Mason J (1970) Copulatory behavior of the crayfish, Pacifastacus trowbridgii (Stimpson). Can J Zool 48:969–976
McBride R, Somarakis S, Fitzhugh G et al (2015) Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish 16:23–57
McLay C, van den Brink A (2016) Crayfish growth and reproduction. In: Longshaw M, Stebbing P (eds) Biology and ecology of crayfish. CRC Press, Boca Raton, pp 62–116
Moore M, Riesch R, Martin R (2016) The predictability and magnitude of life-history divergence to ecological agents of selection: a meta-analysis in livebearing fishes. Ecol Lett 19:435–442
Nichols J, Kendall W (1995) The use of multi-state capture-recapture models to address questions in evolutionary ecology. J Appl Stat 22:835–846
Nichols J, Hines J, Lebreton J et al (2000) Estimation of contributions to population growth: a reverse-time capture–recapture approach. Ecology 81:3362–3376
O'Brien D, Min T, Larsen T et al (2008) Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr Biol 18:R155–R156
Pianka E (1970) On r- and K-Selection. Am Nat 104:592–597
Pippitt M (1977) Mating behavior of the crayfish Orconectes nais (Faxon, 1885) (Decapoda, Astacoidea). Crustaceana 32:265–271
Poff N (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J N Am Benthol Soc 16:391–409
Poulson T, White W (1969) The cave environment. Science 165:971–981
Prins R (1968) Comparative ecology of the crayfishes Orconectes rusticus and Cambarus tenebrosus in Doe Run, Meade County, Kentucky. Int Rev Hydrobiol 53:667–714
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rankin D, Kokko H (2007) Do males matter? The role of males in population dynamics. Oikos 116:335–348
Rétaux S, Casane D (2013) Evolution of eye development in the darkness of caves: adaptation, drift, or both? EvoDevo 4:26
Rideout R, Burton M, Rose G (2000) Observations on mass atresia and skipped spawning in northern Atlantic cod, from Smith Sound, Newfoundland. J Fish Biol 57:1429–1440
Robinet C, Lance D, Thorpe K et al (2008) Dispersion in time and space affect mating success and Allee effects in invading gypsy moth populations. J Anim Ecol 77:966–973
Rogowski D, Sitko S, Bonar S (2013) Optimizing control of invasive crayfish using life-history information. Freshw Biol 58:1279–1291
Sarojini R, Nagabhushanam R, Fingerman M (1995) Naphthalene-induced atresia in the ovary of the crayfish, Procambarus clarkii. Ecotoxicol Environ Saf 31:76–83
Schneider K, Christman M, Fagan W (2011) The influence of resource subsidies on cave invertebrates: results from an ecosystem-level manipulation experiment. Ecology 92:765–776
Simon K, Benfield E (2001) Leaf and wood breakdown in cave streams. J N Am Benthol Soc 20:550–563
Simon K, Benfield E, Macko S (2003) Food web structure and the role of epilithic biofilms in cave streams. Ecology 84:2395–2406
Soares D, Niemiller M (2013) Sensory adaptations of fishes to subterranean environments. Bioscience 63:274–283
Tessier A, Woodruff P (2002) Trading off the ability to exploit rich versus poor food quality. Ecol Lett 5:685–692
Tessier A, Leibold M, Tsao J (2000) A fundamental trade-off in resource exploitation by Daphnia and consequences to plankton communities. Ecology 81:826–841
Tökölyi J, Bradács F, Hóka N et al (2016) Effects of food availability on asexual reproduction and stress tolerance along the fast–slow life history continuum in freshwater hydra (Cnidaria: Hydrozoa). Hydrobiologia 766:121–133
Trippel E, Harvey H (1989) Missing opportunities to reproduce: an energy dependent or fecundity gaining strategy in white sucker (Catostomus commersoni)? Can J Zool 67:2180–2188
Venarsky M, Huntsman B (2018) Food webs in caves. In: Moldovan OT, Kovác L, Halse S (eds) Cave ecology. Springer, Cham, pp 309–328
Venarsky M, Benstead J, Huryn A (2012a) Effects of organic matter and season on leaf litter colonisation and breakdown in cave streams. Freshw Biol 57:773–786
Venarsky M, Huryn A, Benstead J (2012b) Re-examining extreme longevity of the cave crayfish Orconectes australis using new mark–recapture data: a lesson on the limitations of iterative size-at-age models. Freshw Biol 57:1471–1481
Venarsky M, Huntsman B, Huryn A et al (2014) Quantitative food web analysis supports the energy-limitation hypothesis in cave stream ecosystems. Oecologia 176:859–869
Venarsky M, Benstead J, Huryn A et al (2018) Experimental detritus manipulations unite surface and cave stream ecosystems along a common energy gradient. Ecosystems 21:629–642
Veran S, Beissinger S (2009) Demographic origins of skewed operational and adult sex ratios: perturbation analyses of two-sex models. Ecol Lett 12:129–143
Villanelli F, Gherardi F (1998) Breeding in the crayfish, Austropotamobius pallipes: Mating patterns, mate choice and intermale competition. Freshw Biol 40:305–315
Vlaming V (1972) Environmental control of teleost reproductive cycles: a brief review. J Fish Biol 4:131–140
Acknowledgements
Funding for this project was provided by the University of Alabama and two State Wildlife Grants entitled “Assessment of population dynamics of cave inhabiting crayfish in Alabama” to A.D.H. and B.R.K (Grant # T-03-02 and T-3-3-2). Data for this project were collected under State of Alabama Department of Conservation Permit 2010000029568680. F.A. thanks the New Mexico State Agricultural Experiment Station for financial support (NM Fitsum-2017H). We are grateful to Horace Clemens and the Southeastern Cave Conservancy for cave access. Thanks to Chau Tran, Jim Godwin, Justin Cook, Tom Heatherly, Dr. Michael Kendrick, Randall Blackwood, Stuart W. McGregor, Lauren Showalter, Mica Junior, Dru Holla, Dr. Tim Wynn, Cameron Craig, Samantha Richter, Chase Moon, Jonathan Hopper, Jessica Hopper, James Ramsey, Dr. Mick Demi, Dr. Dan Nelson, Dr. Brook Fluker, Derrick Wells, and Dr. Michael Sandel for their assistance in the field and laboratory. This manuscript was improved by helpful comments by Dr. Michael Sandel. The authors of this manuscript have no conflicts of interest to report.
Author information
Authors and Affiliations
Contributions
BMH, MPV, FA and CLC conceived the ideas; MPV, ADH, BRK, and JPB designed methodology; BMH, MPV, ADH, BRK, and JPB collected data; BMH and FA analyzed the data; BMH led writing of the manuscript; all authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huntsman, B.M., Venarsky, M.P., Abadi, F. et al. Evolutionary history and sex are significant drivers of crayfish demography in resource-limited cave ecosystems. Evol Ecol 34, 235–255 (2020). https://doi.org/10.1007/s10682-019-10029-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-10029-w