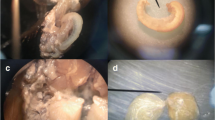
Abstract
Reproduction in less favourable conditions requires genetic adaptation and/or behavioural plasticity of the organism. In order to determine the effects of these mechanisms on environment-associated variability in polyandry, a phenomenon related to reproductive success, we explored the frequency of copulations in females of nuptial gift-giving bush-cricket Pholidoptera griseoaptera (Orthoptera: Tettigoniidae) using a laboratory experiment. In a factorial design, we reared two populations originating from contrasting altitudes in two temperature treatment conditions. After 3 weeks for possible copulations in established mating groups, females (n = 108) contained between 0 and 15 spermatodoses (a proxy for the number of copulations) in their spermatheca. The mean number of spermatodoses per female did not differ either between lowland and highland populations or between warm and cold treatments. Thus, we did not observe main effects of these two factors on adaptation or plasticity. In contrast, the frequency of copulations was significantly affected by female size as log(number of spermatodoses) increased by 0.41 ± 0.27 per each 0.1 mm of pronotum length. However, interactions between the body size (the trait that predicts females’ quality for reproduction) with environmental factors revealed that larger females originating from the highland population and larger females reared in cold treatment copulated more often than smaller ones, whereas females’ size did not affect copulation frequency in the lowland population or in warm treatment. It suggests stronger competition among females in harsher environmental conditions, whereas effect sizes of interaction terms showed that observed mating behaviour expressed a similar extent of genetic and plastic responses to female size. This first observation of environment-associated body size-dependent mating behaviour suggests the interplay of sexual and natural selection in a nuptial gift-giving species.

Similar content being viewed by others
References
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Bates B, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-6. http://cran.r-project.org/package=lme4
Baur B, Baur H, Roesti C, Roesti D (2006) Die Heuschrecken der Schweiz. Haupt, Bern
Berner D, Körner C, Blanckenhorn WU (2004) Grasshopper populations across 2000 m of altitude: is there life history adaptation? Ecography 27:733–740
Best AR, Lewis Z, Hurst GDD, Lizé A (2012) Thermal environment during and outside courtship jointly determine female remating rate in Drosophila melanogaster. Anim Behav 83:1483–1490
Birkhead TR, Hunter FM (1990) Mechanisms of sperm competition. Trends Ecol Evol 5:48–52
Bretman A, Tregenza T (2005) Measuring polyandry in wild populations: a case study using promiscuous crickets. Mol Ecol 14:2169–2179
Brown WD (2008) Size-biased mating in both sexes of the black-horned tree cricket, Oecanthus nigricornis Walker (Orthoptera: Gryllidae: Oecanthinae). J Insect Behav 21:130–142
Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT (2008) Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322:258–261
Cornwallis CK, Uller T (2010) Towards an evolutionary ecology of sexual traits. Trends Ecol Evol 25:145–152
Detzel P (1998) Die Heuschrecken Baden-Württembergs. Ulmer, Stuttgart
Diekötter T, Baveco H, Arens P, Rothenbuhler C, Billeter R, Csencsics D, De Filippi R, Hendrickx F, Speelmans M, Opdam P, Smulders MJM (2010) Patterns of habitat occupancy, genetic variation and predicted movement of a flightless bush cricket, Pholidoptera griseoaptera, in an agricultural mosaic landscape. Landscape Ecol 25:449–461
Fedorka KM, Mousseau TA (2002) Material and genetic benefits of female multiple mating and polyandry. Anim Behav 64:361–367
Fisher DN, Doff RJ, Price TAR (2013) True polyandry and pseudopolyandry: Why does a monandrous fly remate? BMC Evol Biol 13:157
Gowaty PA (2013) Adaptively flexible polyandry. Anim Behav 86:877–884
Grazer VM, Martin OY (2012) Elevated temperature changes female costs and benefits of reproduction. Evol Ecol 26:625–637
Gwynne DT (1984) Sexual selection and sexual differences in Mormon crickets (Orthoptera: Tettigoniidae, Anabrus simplex). Evolution 38:1011–1022
Gwynne DT (2001) Katydids and bush-crickets: reproductive behavior and evolution of the Tettigoniidae. Cornell University Press, Ithaca and London
Gwynne DT (2008) Sexual conflict over nuptial gifts in insects. Ann Rev Entomol 53:83–101
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Honěk A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Hosken DJ, Garner TWJ, Tregenza T, Wedell N, Ward PI (2003) Superior sperm competitors sire higher-quality young. Proc R Soc B: Biol Sci 270:1933–1938
Ingrisch S, Köhler G (1998) Die Heuschrecken Mitteleuropas. Die Neue Brehm Bücherei 629. Westarp Wissenschaften, Magdeburg
Jarčuška B, Kaňuch P (2014) Female bush-crickets, Pholidoptera griseoaptera, that have received smaller ejaculates show a higher mating rate in the field. J Insect Behav 27:411–418
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Kaňuch P, Jarčuška B, Schlosserová D, Sliacka A, Paule L, Krištín A (2012) Landscape configuration determines gene flow and phenotype in a flightless forest-edge ground-dwelling bush-cricket, Pholidoptera griseoaptera. Evol Ecol 26:1331–1343
Kaňuch P, Kiehl B, Low M, Cassel-Lundhagen A (2013) On variation of polyandry in the bush-cricket Metrioptera roeselii in northern Europe. J Insect Sci 13:16
Katsuki M, Miyatake T (2009) Effects of temperature on mating duration, sperm transfer and remating frequency in Callosobruchus chinensis. J Insect Physiol 55:113–116
Kemp DJ (2012) Costly copulation in the wild: mating increases the risk of parasitoid-mediated death in swarming locusts. Behav Ecol 23:191–194
Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM (2012) Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol Ecol 26:1101–1118
Knell RJ, Webberley KM (2004) Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol Rev 79:557–581
Kvarnemo C, Simmons LW (2013) Polyandry as a mediator of sexual selection before and after mating. Phil Trans R Soc B: Biol Sci 368(1613):20120042
Lehmann GU (2012) Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Frontiers Zool 9(1):9
Lewis SM, Vahed K, Koene JM, Engqvist L, Bussière LF, Perry JC, Gwynne D, Lehmann GUC (2014) Emerging issues in the evolution of animal nuptial gifts. Biol Lett 10:20140336
Mani MS (1968) Ecology and biogeography of high altitude insects. Dr. W. Jung N. V Publishers, The Hague
Møller AP (1992) Frequency of female copulation with multiple mates and sexual selection. Am Nat 139:1089–1101
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Parker GA, Simmons LW (1989) Nuptial feeding in insects: theoretical models of male and female interests. Ethology 82:3–26
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. The John Hopkins University Press, Baltimore and London
Pinzone CA, Dyer KA (2013) Association of polyandry and sex-ratio drive prevalence in natural populations of Drosophila neotestacea. Proc R Soc Lond B Biol Sci 280(1769):20131397
Plesnar-Bielak A, Skrzynecka AM, Prokop ZM, Radwan J (2012) Mating system affects population performance and extinction risk under environmental challenge. Proc R Soc B: Biol Sci 279:4661–4667
Price TA, Bretman A, Gradilla AC, Reger J, Taylor ML, Giraldo-Perez P, Campbell A, Hurst GDD, Wedell N (2014) Does polyandry control population sex ratio via regulation of a selfish gene? Proc R Soc Lond B Biol Sci 281(1783):20133259
R Core Team (2014) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/
Samietz J, Salser MA, Dingle H (2005) Altitudinal variation in behavioural thermoregulation: local adaptation vs. plasticity in California grasshoppers. J Evol Biol 18:1087–1096
Sexton JP, Hangartner SB, Hoffmann AA (2014) Genetic isolation by environment or distance: which pattern of gene flow is most common? Evolution 68:1–15
Simmons LW, Beveridge M, Kennington WJ (2007) Polyandry in the wild: temporal changes in female mating frequency and sperm competition intensity in natural populations of the tettigoniid Requena verticalis. Mol Ecol 16:4613–4623
Snook RR (2014) The evolution of polyandry. In: Shuker DM, Simmons LW (eds) The evolution of insect mating systems. Oxford Univ. Press, Oxford, pp 159–180
Taylor ML, Price TA, Wedell N (2014) Polyandry in nature: a global analysis. Trends Ecol Evol 29:376–383
Tregenza T, Wedell N (1998) Benefits of multiple mates in the cricket Gryllus bimaculatus. Evolution 52:1726–1730
Uma R, Sevgili H (2015) Spermatophore allocation strategy over successive matings in the bushcricket Isophya sikorai (Orthoptera Phaneropterinae). Ethol Ecol Evol 27:129–147
Vahed K (1998) The function of nuptial feeding in insects: a review of empirical studies. Biol Rev 73:43–78
Vahed K (2003) Structure of spermatodoses in shield-back bushcrickets (Tettigoniidae, Tettigoniinae). J Morphol 257:45–52
Vahed K (2006) Larger ejaculate volumes are associated with a lower degree of polyandry across bushcricket taxa. Proc R Soc B: Biol Sci 273:2387–2394
Välimäki P, Kaitala A (2010) Properties of male ejaculates do not generate geographical variation in female mating tactics in a butterfly Pieris napi. Anim Behav 79:1173–1179
Wedell N (1993) Spermatophore size in bushcrickets: comparative evidence for nuptial gifts as sperm protection devices. Evolution 47:1203–1212
Wedell N, Ritchie MG (2004) Male age, mating status and nuptial gift quality in a bushcricket. Anim Behav 67:1059–1065
Whitman DW (2008) The significance of body size in the Orthoptera: a review. J Orthop Res 17:117–134
Winkler JD, Van Buskirk J (2012) Influence of experimental venue on phenotype: multiple traits reveal multiple answers. Funct Ecol 26:513–521
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgments
We thank Karim Vahed, Tom Reader and two anonymous reviewers for providing us with valuable comments on a previous version of the manuscript. This study was funded by the Slovak Scientific Grant Agency VEGA (Grant number 2/0061/15).
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter Kaňuch and Benjamín Jarčuška contributed equally to writing of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaňuch, P., Jarčuška, B., Kovács, L. et al. Environmentally driven variability in size-selective females’ mating frequency of bush-cricket Pholidoptera griseoaptera . Evol Ecol 29, 787–797 (2015). https://doi.org/10.1007/s10682-015-9784-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-015-9784-5