Abstract
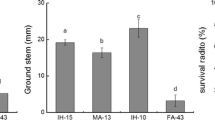
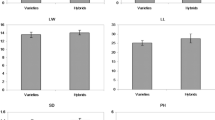
For cross-pollination trees, the optimal breeding method is hybridization. Tree heterosis is commonly present and is the main research focus in tree crossbreeding. Salt stress and interspecific hybridization may lead to DNA methylation changes. The study crossed Fraxinus mandshurica (female parent) with Fraxinus velutina (male parent) to obtain interspecific F1 hybrid progenies that could obtain the good characters of parents. The results showed that growth and survival rate of the interspecific hybrid progenies (F1 hybrids of F. mandshurica × F. velutina) were significantly higher than those of intraspecific open pollinated plants from parental F. mandshurica and F. velutina. Salt tolerance and cytosine methylation in interspecific F1 hybrids and the intraspecific open pollinated plants from parents were examined. Membrane permeability, ROS and antioxidant activity, malondialdehyde, and photosynthesis were measured after salt treatment and genomic methylation was analyzed using a methylation-sensitive amplified polymorphism protocol. F1 hybrids exhibited heterosis for growth in normal as well as high salt conditions. DNA methylation in the F1 hybrids was lower than the intraspecific open pollinated plants from parents. Salt treatments changed DNA methylation patterns in F1 hybrids. Genomic DNA of the intraspecific open pollinated plants from parents had internal cytosine methylation (average of 13.22 %), whereas F1 hybrid seedlings had external cytosine methylation (average of 7.34 %). Such changes in DNA methylation patterns in F1 hybrids suggest a connection between salt tolerance and epigenetic mechanisms in plants. We observed that alteration of DNA methylation was closely correlated with the adaptation to the salt stress and provided epigenetic mechanisms of salt tolerance in the interspecific hybridization of trees.







Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DHAR:
-
Dehydroascorbate reductase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- mC:
-
Methylcytosine
- MDAR:
-
Monodehydroascorbate reductase
- POD:
-
Peroxidises
- ROS:
-
Reactive oxygen species
- SE:
-
Standard error
- SOD:
-
Superoxide dismutase
- Pn:
-
Net photosynthetic rate
- Gs:
-
Stomatal conductance
- Ci:
-
Intercellular CO2 concentration
- Tr:
-
Transpiration rate
- MSAP:
-
Methylation-sensitive amplified polymorphism
References
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhem, pp 273–286
Amako K, Chen GX, Asada K (1994) Separate assay specific for ascorbate peroxidase and guaiacol peroxidase and for chloroplastic and cytosolic isoenzymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Apel K, Hir H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Ashikawa I (2001) Surveying CpG methylation at 5′-CCGG in the genomes of rice cultivars. Plant Mol Biol 45:31–39
Bilichak A, Ilnystkyy Y, Hollunder J, Kovalchuk I (2012) The progeny of Arabidopsis thaliana plants exposed to salt exhibit changes in DNA methylation, histone modifications and gene expression. PLoS ONE 7(1):e30515. doi:10.1371/journal.ppat.1004735
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Bohnert HJ, Jensen RG (1996) Metabolic engineering for increased salt tolerance. Aust J Plant Physiol 23:661–667
Boyko A, Kovalchuk I (2011) Genome instability and epigenetic modification -heritable responses to environmental stress? Curr Opin Plant Biol 14:260–266
Boyko A, Hudson D, Bhomkar P, Kathiria P, Kovalchuk I (2006) Increase of homologous recombination frequency in vascular tissue of Arabidopsis plants exposed to salt stress. Plant Cell Physiol 47:736–742
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bräutigam K, Vining KJ, Lafon-Placette C et al (2013) Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol Evol 3:399–415
Cao X, Jacobsen SE (2002) Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12:1138–1144
Causevic A, Gentil MV, DelaunayA El-SoudWA, GarciaZ Pannetier C, Brignolas F, Hagège D, Maury S (2006) Relationship between DNA methylation and histone acetylation levels, cell redox and cell differentiation states in sugarbeet lines. Planta 224:812–827
Chan SW, Henderson IR, Jacobsen SE (2005) Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet 6:351–360
Chinnusamy V, Zhu JK (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12:133–139
DaCosta M, Huang BR (2007) Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J Am Soc Hortic Sci 132:319–326
Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize. Plant Physiol 114:1031–1037
Dyachenko OV, Zakharchenko NS, Shevchuk TV, Bohnert HJ, Cushman JC, Buryanov YI (2006) Effect of hypermethylation of CCWGG sequences in DNA of Mesembryanthemum crystallinum plants on their adaptation to salt stress. Biochemistry (Moscow) 71:461–465
El-Mashad AA, Mohamed HI (2012) Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249(3):625–635
Farmer EE, Mueller MJ (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Annu Rev Plant Biol 64:429–450
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Grant-Downton R, Dickinson H (2005) Epigenetics and its implications for plant biology. 1. The epigenetic network in plants. Ann Bot 96:1143–1164
Grant-Downton RT, Dickinson HG (2006) Epigenetics and its implications for plant biology 2. The ‘epigenetic epiphany’: epigenetics, evolution and beyond. Ann Bot 97(1):11–27
Griffin JR, Critchfield WB (1972) The distribution of forest trees in California. Research paper PSW-82. USDA Forest Service, Pacific Southwest Forest and Range Experiment Station, Berkeley, CA
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hepburn AG, Wade M, Fraser RSS (1985) Present and future prospects for exploitation of resistance in crop protection by novel means. Mech Resist Plant Dis Adv Agric Biotechnol 17:425–452
Hernandez M, Fernandez-Garcia N, Diaz-Vivancos P, Olmos E (2010) A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J Exp Bot 61(2):521–535
Hernández JA, Jiménez A, Mullineaux PM, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862
Hooftman DAP, De Jong MJ, Oostermeijer JGB, Den Nijs HCM (2007) Modelling the long-term consequences of crop–wild relative hybridization: a case study using four generations of hybrids. J Appl Ecol 44:1035–1045
Hooftman DAP, Hartman Y, Oostermeijer JGB, Den Nijs HCM (2009) Existence of vigorous lineages of crop–wild hybrids in lettuce under field conditions. Environ Biosaf Res 4:203–217
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hu LJ, Uchiyama K, Shen HL, Saito Y, Tsuda Y, Ide Y (2008) Nuclear DNA microsatellites reveal genetic variation but a lack of phylogeographical structure in an endangered species, Fraxinus mandshurica, across North-east China. Ann Bot 102:195–205
Hua Y, Chen XF, Xiong JH, Zhang YP, Zhu YG (2005) Isolation and analysis of differentially methylated fragment CIDM7 in rice induced by cold stress. Heredits 27(4):595–600
Karan R, DeLeon T, Biradar H, Subudhi PK (2012) Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 7(6):e40203. doi:10.1371/journal.ppat.1004735
King IP, Law CN, Cant KA, Orford SE, Reader SM, Miller TE (1997) Tritipyrum, a potential new salt-tolerant cereal. Plant Breed 116:127–132
Kočová M, Holá D, Wilhelmová N, Rothová O (2009) The influence of low-temperature on the photochemical activity of chloroplasts and activity of antioxidant enzymes in maize leaves. Biol Plant 53:475–483
Kosugi H, Kikugawa K (1985) Thiobarbituric acid reaction of aldehydes and oxidized lipids in glacial acetic acid. Lipids 20(12):915–921
Kovalchuk I, Kovalchuk O, Kalck V, Boyko V, Filkowski J, Heinlein M, Hohn B (2003) Pathogen-induced systemic plant signal triggers DNA rearrangements. Nature 423:760–762
Kovarik A, Koukalova B, Bezdek M, Opatrn Z (1997) Hypermethylation of tobacco heterochromatic loci in response to osmotic stress. Theor Appl Genet 95:301–306
Labra M, Ghiani A, Citterio S, Sgorbati S, Sala F, Vannini C, Ruffini-Castiglione M, Bracale M (2002) Analysis of cytosine methylation pattern in response to water deficit in pea root tips. Plant Biol (Stuttgart) 4:694–699
Li B, Howe GT, Wu R (1998) Developmental factors responsible for heterosis in aspen hybrids (Populus tremuloides × P. tremula. Tree Physiol 18(1):29–36
Lima ALS, DaMatta FM, Pinheiro HA, Totola MR, Loureiro ME (2002) Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ Exp Bot 47:239–247
Liu B, Wendel JF (2000) Retrotransposon activation followed by rapid repression in introgressed rice plants. Genome 43:874–880
Liu H, Bauer LS, Gao R, Zhao T, Petrice TR, Haack RA (2003) Exploratory survey for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), and its natural enemies in China. Great Lakes Entomol 36:191–204
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oriza sativa L.) cultivar differing in salinity resistance. Ann Bot 78:389–398
Ma YH, Ma FW, Zhang JK, Li MJ, Wang YH, Liang D (2008) Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci 157:761–766
Maribel L, Dionisio-Sese Satoshi T (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Meir S, Philosoph-hadas S, Aharoni N (1992) Ethylene-increased accumulation of fluorescent lipid-peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci 117:128–132
Mirouze M, Paszkowski J (2011) Epigenetic contribution to stress adaptation in plants. Curr Opin Plant Biol 14:267–274
Misra N, Gupta AK (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J Plant Physiol 163:11–18
Mittler R, Merquiol E, Hallak-Herr E, Rachmilevitch S, Kaplan A, Cohen M (2001) Living under a “dormant” canopy: a molecular acclimation mechanism of the desert plant Retama raetam. Plant J 25:407–416
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium andreactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140
Natcheva R, Cronberg N (2007) Maternal transmission of cytoplasmic DNA in interspecific hybrids of peat mosses, Sphagnum (Bryophyta). J Evol Biol 20:1613–1616
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Parida AK, Das AB, Mittra B (2003) Effect of NaCl stress on the structure, pigment complex composition, and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts. Photosynthetica 41:191–200
Pnueli L, Hongjian L, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34:187–203
Porebski S, Bailey LG, Baum BR (1997) Modification of a cTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Portis E, Acquadro A, Comino C, Lanteri S (2004) Analysis of DNA methylation during germination of peper (Capsicum annuum L) seeds using ethylation-sensitive amplification polymorphism (MSAP). Plant Sci 166(1):169–178
Puckette MC, Weng H, Mahalingam R (2007) Physiological and biochemical responses to acute ozone-induced oxidative stress in Medicago truncatula. Plant Physiol Biochem 45(1):70–79
Queiroz CGS, Alonso A, Mares-Guia M, Magalhaes AC (1998) Chilling-induced changes in membrane fluidity and antioxidant enzyme activities in Coffea arabica L. roots. Biol Plant 41:403–413
Rangwala SH, Richards EJ (2004) The value-added genome: building and maintaining genomic cytosine methylation landscapes. Curr Opin Genet Dev 14:686–691
Rapp RA, Wendel JF (2005) Epigenetics and plant evolution. New Phytol 168:81–91
Reyna-Lopez GE, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 253:703–710
Ruiz GL, Cervera MT, Martínez-Zapater JM (2005) DNA methylation increases throughout Arabidopsis development. Planta 222(2):301–306
Salmon A, Ainouche ML, Wendel JF (2005) Genetic and epigenetic consequences of recent hybridization and polyploidy in Spartina (Poaceae). Mol Ecol 14:1163–1175
SarangaY Zamir D, Marani A, Rudich J (1991) Breeding tomatoes for salt tolerance—field-evaluation of Lycopersicon germplasm for yield and dry-matter production. J Am Soc Hortic Sci 116:1067–1071
Shan X, Liu Z, Dong Z, Wang Y, Chen Y, Lin X, Long L, Han F, Dong Y, Liu B (2005) Mobilization of the active MITE transposons mPing and Pong in rice by introgression from wild rice (Zizania latifolia Griseb.). Mol Biol Evol 22:976–990
Shepherd M, Kasem S, Lee DJ, Henry R (2008) Mapping species differences for adventitious rooting in a Corymbia torelliana × Corymbia citriodora subspecies variegata hybrid. Tree Genet Genomes 4(4):715–725
Shivaprasad PV, Dunn RM, Santos BACM, Bassett A, Baulcombe DC (2011) Extraordinary transgressive phenotypes of hybrid tomato are influenced by epigenetics and small silencing RNAs. The EMBO J 31:257–266
Smith I (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79:1044–1047
Song Y, Ji D, Li S, Wang P, Li Q, Xiang F (2012) The dynamic changes of DNA methylation and histone modifications of salt responsive transcription factor genes in soybean. PLoS ONE 7(7):e41274
Springer NM, Robert M (2007) Stupar Allelic variation and heterosis in maize: how do two halves make more than a whole? Genome Res 17:264–275
Tal M, Shannon MC (1983) Salt tolerance in two wild relatives of the cultivated tomato: responses of Lycopersican esculentum, L. cheesmani, L. peruvianum, Solanum pennelli, and F1 hybrids of high salinity. Aust J Plant Physiol 10:109–117
Tavakkoli E, Rengasamy P, McDonald GK (2010) High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 61(15):4449–4459
Tozlu I, Moore GA, Guy CL (2000) Regulation of growth and differential tissue dry mass accumulation by Citrus grandis, Poncirus trifoliata, and their F1 under salinized and non-salinized environments. Aust J Plant Physiol 27:27–33
Tsaftaris AS, Kafka M (1998) Mechanisms of heterosis in crop plants. J Crop Prod 1:95–111
Wang R, Chen S, Deng L, Fritz E, Hüttermann A, Polle A (2007) A Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees-Struct Funct 21:581–591
Xiong LZ, Xu CG, Shagi-Maroof MA, Zhang Q (1999) Patterns of cytosine methyaltion in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Mol Genet Genomics 261:439–446
Xu L, Han L, Huang B (2011) Antioxidant enzyme activities and gene expression in drought-stressed Kentucky bluegrass. J Am Soc Hortic Sci 136:247–255
Yan H, Kikuchi S, Neumann P, Zhang W, Wu Y, Chen F, Jiang J (2010) Genome-wide mapping of cytosine methylation revealed dynamic DNA methylation patterns associated with genes and centromeres in rice. Plant J 63:353–365
Zhou R, Zhao H (2004) Seasonal pattern of antioxidant enzyme system in the roots of perennial forage grasses grown in alpine habitat, related to freezing tolerance. Physiol Plant 121:399–408
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu JK (2008) Epigenome sequencing comes of age. Cell 133:395–397
Zhu Z, Chen J, Zheng HL (2012) Physiological and proteomic characterization of salt tolerance in a mangrove plant, Bruguiera gymnorrhiza (L.) Lam. Tree Physiol 32(11):1378–1388
Zlatev ZS, Lidon FC, Ramalho JC, Yordanov IT (2006) Comparison of resistance to drought of three bean cultivars. Biol Plant 50:389–394
Acknowledgments
This work was financially supported by The National Forestry Science and Technology Support Program (2012BAD01B0503), The Innovation Project of State Key Laboratory of Tree Genetics and Breeding (Northeast Forestry University) (2013B04) and the National Natural Science Foundation of China (NO: 31270697) and Harbin Science and Technology Innovation Funds (RC2011QN002051). We also thank the editor and anonymous reviewers for many detailed and helpful comments that improved the quality of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Data archiving statement
The research performed in the present study did not produce any data on nucleic acid or protein sequences, genetic maps, SNPs or gene expression that could be deposited in the public databases. Data of enzyme assays and physiological parameters is stored at Northeast Forestry University, Harbin (http://www.nefu.edu.cn/).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, FS., Li, LL., Liang, NS. et al. Salt tolerance and alterations in cytosine methylation in the interspecific hybrids of Fraxinus velutina and Fraxinus mandshurica . Euphytica 205, 721–737 (2015). https://doi.org/10.1007/s10681-015-1432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1432-1