Abstract
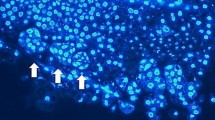
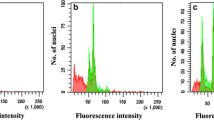
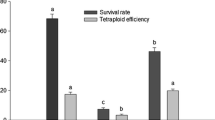
An efficient protocol for colchicine mediated production of in vitro autotetetraploids from Ponkan mandarin using cell suspension cultures is described. Cells were treated with 1 g l−1 colchicine for 4 or 8 days before transfer into solid EME medium supplemented with 5% maltose. Colchicine treated cells were placed in medium with or without an overlay of 1:2 medium–mixture of liquid 0.6 M BH3 medium and 0.15 M EME + maltose liquid media. It was observed that modifying the immediate cell environment by addition of the liquid overlay played a positive role in cell differentiation and subsequent plant regeneration. Ploidy levels were determined with a flow cytometer and confirmed by chromosome staining using the enzymatic maceration method. A large number of non-chimeric autotetraploids were generated using this method. Such plants have great value in a breeding program for the development of seedless triploid citrus, as very few available tetraploid breeding parents are easy to peel.



Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- MT:
-
Murashige and Tucker
- UV:
-
Ultraviolet
References
Attree SM, Pomeroy MK, Fowke LC (1992) Manipulation of conditions for the culture of somatic embryos of white spruce for improved triacylglycerol biosynthesis and desiccation tolerance. Planta 187:395–404
Barrett HC (1974) Colchicine-induced polyploidy in citrus. Bot Gaz 135:29–34
Blakeslee FA, Avery AG (1937) Methods of inducing doubling of chromosome in plants. J Hered 25:80–108
Brisibe EA, Miyake H, Taniguchi T, Maeda E (1994) Abscissic acid and high osmoticum regulation of development and storage reserve accumulation in sugarcane somatic embryos. Jpn J Crop Sci 63:689–698
Esen A, Soost RK (1973) Seed development in Citrus with special reference to 2× × 4× crosses. Am J Bot 60:448–452
Finkelstein RR, Crouch ML (1986) Rapeseed embryo development in culture on high osmoticum is similar to that in seeds. Plant Physiol 81:907–912
Fujii JAA, Slade D, Olsen R, Ruzin SE, Redenbaugh K (1990) Alfalfa somatic embryo maturation and conversion to plants. Plant Sci 72:93–100
Gmitter FG, Ling XB (1991) Embryogenesis in vitro and nonchimeric tetraploid plant-recovery from underdeveloped citrus ovules treated with colchicine. J Am Soc Hort Sci 116:317–321
Gmitter FG, Ling XB, Cai CY, Grosser JW (1991) Colchicine-induced polyploidy in citrus embryogenic cultures, somatic embryos, and regenerated plantlets. Plant Sci 74:135–141
Grosser JW, Chandler JL (2004) Production of twelve new allotetraploid somatic hybrid citrus breeding parents with emphasis on late maturity and cold-hardiness. J Am Pom Soc 58:21–28
Grosser JW, Gmitter FG (1990) Protoplast fusion and citrus improvement. Plant Breed Rev 8:339–374
Grosser JW, Gmitter FG (2005) Thinking outside the cell—applications of somatic hybridization and cybridization in crop improvement, with citrus as a model. In Vitro Cell Dev Biol Plant 41:220–225
Grosser JW, Gmitter FG, Louzada ES, Chandler JL (1992) Production of somatic hybrid and autotetraploid breeding parents for seedless citrus development. HortScience 27:1125–1127
Grosser JW, Ollitrault P, Olivares-Fuster O (2000) Somatic hybridization in citrus: an effective tool to facilitate variety improvement. In Vitro Cell Dev Biol Plant 36:434–449
Grosser JW, Deng XX, Goodrich RM (2007) Somaclonal variation in sweet orange. In: Khan IA (ed) Citrus genetics, breeding and biotechnology. CAB International, UK, pp 219–234
Grzebelus E, Adamus A (2004) Effect of anti-mitotic agents on development and genome doubling of gynogenic onion (Allium cepa L.) embryos. Plant Sci 167:569–574
Hamill SD, Smith MK, Dodd WA (1992) In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Aust J Bot 40:887–896
Hansen AL, Gertz A, Joersbo M, Andersen SB (2000) Chromosome doubling in vitro with amiprophos-methyl in Beta vulgaris ovule culture. Acta Agric Scand Sect B Soil Plant Sci 50:89–95
James DJ, Mackenzie KAD, Malhotra SB (1987) The induction of hexaploidy in cherry rootstocks using in vitro regeneration techniques. Theor Appl Genet 73:589–594
Jaskani MJ, Hassan S, Bashir MA, Khan IA (1996) Morphological descriptions of citrus colchiploids. Proc Int Soc Citriculture 8:37
Juarez J, Aleza P, Olivares-Fuster O, Navarro L (2004) Recovery of tetraploid clementine plants (Citrus clementina Hort. Ex Tan.) by in vitro colchicine treatment of shoot tips. Proc Int Soc Citriculture 10:151–154
Kadota M, Niimi Y (2002) In vitro induction of tetraploid plants from a diploid Japanese pear cultivar (Pyrus pyrifolia N. cn. Hosui). Plant Cell Rep 21:282–286
Kao KN, Michayluck MR (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at very low population density in liquid medium. Planta 126:105–110
Kurata N, Omura T (1978) Karyotype analysis in rice. A new method for identifying all chromosome pairs. Jpn J Genet 4:251–255
Li DD, Shi W, Deng XX (2002) Agrobacterium-mediated transformation of embryonic calluses of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Rep 21:153–156
Lyrene P, Perry JL (1982) Production and selection of blueberry polyploid in vitro. J Hered 73:377–378
Merkel SA, Parrott WA, Flinn BS (1995) Morphogenic aspects of somatic embryogenesis. In: Thorpe TA (ed) In vitro Embryogenesis in Plants. Kluwer Academic Publishers, Netherlands
Murashige T, Tucker DPH (1969) Growth factor requirement of citrus tissue culture. Proc Int Citrus Symp 3:1151–1161
Niedz RP, Hyndman SE, Wynn ET, Bausher MG (2002) Normalizing sweet orange (C. sinensis L. Osbeck) somatic embryogenesis with semipermeable membranes. In Vitro Cell Dev Biol Plant 38:552–557
Notsuka K, Tsuru T, Shiraishi M (2000) Induced polyploidy in grapes via in vitro chromosome doubling. J Jpn Soc Hort Sci 69:543–551
Oiyama I (1992) Studies on polyploidy breeding in citrus with special reference to the production of tetraploid breeding. Bull Fruit Tree Res Station 3:68
Ollitrault P, Vanel F, Froelicher Y, Dambier D (2000) Creation of triploid citrus hybrids by electrofusion of haploid and diploid protoplasts. Acta Hort 535:191–198
Reforgiato Recupero G, Russo G, Recupero S (2005) New promising citrus triploid hybrids selected from crosses between monoembryonic diploid female and tetraploid male parents. HortScience 40:516–520
Stewart FC, Mapes MO, Mears K (1958) Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am J Bot 45:705–708
Vardi A, Galun E (1988) Recent advances in protoplast culture of horticultural crops: citrus. Sci Hort 37:217–230
Wu J, Mooney P (2002) Autotetraploid tangor plant regeneration from in vitro citrus somatic embryogenic callus treated with colchicine. Plant Cell Tissue Organ Cult 70:99–104
Yahata M, Kunitake H, Yasuda K, Yamashita K, Komatsu H, Matsumoto R (2006) Production of sexual hybrid progenies for clarifying the phylogenic relationship between Citrus and Citropsis species. J Am Soc Hort Sci 131:764–769
Yamamoto M, Abkenar AA, Matsumoto R, Kubo T, Tominaga S (2008) CMA staining analysis of chromosomes in several species of Aurantioideae. Genet Resour Crop Evol 55:1167–1173
Zhang J, Zhang M, Deng XX (2007) Obtaining autotetraploids in vitro at a high frequency in Citrus sinensis. Plant Cell Tissue and Organ Cult 89:211–216
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutt, M., Vasconcellos, M., Song, K.J. et al. In vitro production of autotetraploid Ponkan mandarin (Citrus reticulata Blanco) using cell suspension cultures. Euphytica 173, 235–242 (2010). https://doi.org/10.1007/s10681-009-0098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-0098-y