Abstract
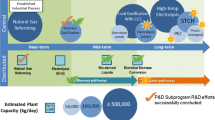
Outotec open cycle (OOC) is a new low-energy process linking together production of hydrogen and sulfuric acid. While sulfuric acid is the world’s most widely produced chemical by mass at approximately 200 Mt/a, the OOC gives the potential for making 4 Mt/a of hydrogen gas as a by-product. H2SO4 manufacture requires a source of sulfur dioxide. 30% of world production of H2SO4 is from the SO2 by-product of pyrometallurgical processing of sulfur containing concentrates of metals such as copper, nickel and zinc. SO2 can also be made by direct combustion of sulfur. In OOC, a divided electrochemical cell is used for SO2-depolarized electrolysis of water. SO2 is fed to the anolyte and converted to H2SO4, while hydrogen gas is produced at the cathode. On the industrial scale, the equipment will be in the form of a membrane electrolyzer assembly or stack. A case is described where the OOC would be connected to a pyrometallurgical plant smelting 1 Mt/a of nickel and copper concentrate, producing 1 Mt/a of H2SO4 and 20 kt/a of hydrogen.






Similar content being viewed by others
References
Anthony, M., Flett, D., & Wells, A. (2004). Sulphur production and usage in the processing of non-ferrous metals. In Sulphur markets—Today and tomorrow. March 21–23, 2004, London, England.
Armstrong, C., et al. (2006). Background paper: The outlook for metals markets prepared for G20 deputies meeting Sydney 2006. Washington: The World Bank Group.
Brecher, L. E., Spewock, S., & Warde, C. J. (1977). The Westinghouse sulfur cycle for the thermochemical decomposition of water. International Journal of Hydrogen Energy, 2, 7–15.
Brecher, L. E., & Wu, C. K. (1975). Electrolytic decomposition of water. U.S. Patent No 3888750, assigned to Westinghouse Electric Corporation, June 10th, 1975.
BREF Document for the Manufacture of Large Volume Inorganic Chemicals Ammonia, Acids and Fertilisers Industries (2007). European Commission. http://eippcb.jrc.es/reference/lvic-aaf.html. Accessed 4 November 2011.
Bryk, P., Ryselin, J., Honkasalo, J., & Malmström, R. (1958). Flash smelting copper concentrates. Journal of Metals, 10, 395–400.
Carty, R., Cox, K., Funk, J., Soliman, M., Conger, W., Brecher, L., et al. (1977). Process sensitivity studies of the Westinghouse sulfur cycle for hydrogen generation. International Journal of Hydrogen Energy, 2, 17–22.
Charit, I., & Murty, K. L. (2010). Structural materials issues for the next generation fission reactors. JOM Journal of the Minerals Metals and Materials Society, 62(9), 67–74.
Forsberg, C. W. (2009). Hydrogen, nuclear energy and the advanced high-temperature reactor. International Journal of Hydrogen Energy, 28, 1073–1081.
Galzim, Q., Mansilla, C., Giaconia, A., Poitou, S., Hinkley, J., Ebbesen, S. D., et al. (2011). A multicriteria approach for evaluating high temperature hydrogen production processes. International Journal of Multicriteria Decision Making, 1(2), 177–204.
Goodwin, F. E. (2006). Zinc and zinc alloys. In Kirk-Othmer encyclopedia of chemical technology (pp. 554–604). London: Wiley.
Gorensek, M. B., Staser, J. A., Stanford, T. G., & Weidner, J. W. (2009). A thermodynamic analysis of the SO2/H2SO4 system in SO2-depolarized electrolysis. International Journal of Hydrogen Energy, 34, 6089–6095.
Gorensek, M. B., & Summers, W. A. (2009). Hybrid sulfur flow sheets using PEM electrolysis and a bayonet decomposition reactor. International Journal of Hydrogen Energy, 34, 4097–4114.
Graf, G. G. (2008). Zinc. In Ullmann’s encyclopedia of industrial chemistry (7th ed.). Weinheim: Wiley.
ICIS Sulphur (2010). http://www.icis.com/staticpages/sulphur_sulphuric_acid_lp.htm. Accessed 4 November 2011.
Jeong, Y. H., & Kazimi, M. S. (2007). Optimization of the hybrid sulfur cycle for nuclear hydrogen generation. Nuclear Technology, 159, 147–157.
Jomard, F., Feraud, J. P., & Caire, J. P. (2008). Numerical modeling for preliminary design of the hydrogen production electrolyzer in the Westinghouse hybrid cycle. International Journal of Hydrogen Energy, 33, 1142–1152.
Juda, W., & Moulton, D. M. (1967). Cheap hydrogen for basic chemicals. Chemical Engineering Progress, 63(4), 59–60.
Kojo, I. V., Jokilaakso, A., & Hanniala, P. (2000). Flash smelting and converting furnaces: A 50 year retrospect. JOM Journal of the Minerals Metals and Materials Society, 48, 57–61.
Kojo, I. V., & Storch, H. (2006). Copper production with Outokumpu flash smelting: An update. In Proceeding of Sohn international symposium advanced processing of metals and materials. Vol 8—International symposium of sulfide smelting, TMS (pp. 225–238).
Kruger, P. (2008). Appropriate technologies for large-scale production of electricity and hydrogen fuel. International Journal of Hydrogen Energy, 33, 5881–5886.
Lu, P. W. T., & Ammon, R. L. (1982). Sulfur dioxide depolarized electrolysis for hydrogen production: development status. International Journal of Hydrogen Energy, 7(7), 563–575.
Mäkinen, T., & Taskinen, P. (2006). The state of the art in nickel smelting: Direct Outokumpu nickel technology. In F. Kongoli & R. Reddy (Eds.) Proceeding of Sohn international symposium advanced processing of metals and materials (Vol. 8, pp. 313–325). Warrendale (PA), TMS.
McLaughlin, D. F., Paletta, S. A., Lahoda, E. J., & Kriel, W. (2006). Revised capital and operating HyS hydrogen production costs. In Proceedings of ICAPP’06. June 4–8, 2006, Reno, USA.
Müller, H. (2008a). Sulfuric acid and sulfur trioxide. In Ullmann’s encyclopedia of industrial chemistry (7th ed.). Weinheim: Wiley.
Müller, H. (2008b). Sulfur dioxide. In Ullmann’s encyclopedia of industrial chemistry (7th ed.). Weinheim: Wiley.
O’Brien, J. A., Hinkley, J. T., Donne, S. W., & Lindquist, S.-E. (2010). The electrochemical oxidation of aqueous sulfur dioxide: A critical review of work with respect to the hybrid sulfur cycle. Electrochimica Acta, 55, 573–591.
Rauser, W. -C., Gasik, M., Peltola, H., Taskinen, P. (2010). Menetelmä vedyn ja rikkihapon valmistamiseksi (A method for producing hydrogen and sulpuric acid). Patent FI 121271B, assigned to Outotec Oyj, September 15th, 2010.
Staser, J. A., & Weidner, J. W. (2009). Effect of water transport on the production of hydrogen and sulfuric acid. Journal of the Electrochemical Society, 159(1), B16–B21.
Steimke, J. L., & Steeper, T. J. (2006). Characterization testing and analysis of single cell SO 2 depolarized electrolyzer (p. 56). Research report WSRC-STI-2006-00120.
Stolten, D., & Krieg, D. (2010). Alkaline electrolysis—Introduction and overview. In D. Stolten (Ed.), Hydrogen and fuel cells fundamentals, technologies and applications (pp. 243–270). Weinheim: Wiley.
Struck, B. D., Junginger, R., Boltersdorf, D., & Gehrmann, J. (1980). The anodic oxidation of sulfur dioxide in the sulfuric acid hybrid cycle. International Journal of Hydrogen Energy, 5, 487–497.
Sulphuric Acid on the Web (2010). http://www.sulphuric-acid.com/sulphuric-acid-on-the-web/Acid%20Plants/Acid_Plant_Index.htm. Accessed 4 November 2011.
Taskinen, P., Metsärinta M.-L., Saxen, B., Penttinen, S., Svens, K., & Kerstiens, B. (2008). Increased productivity of zinc roasters and SO2 quality. In Proceedings of international conference on lead and zinc 2008 (pp. 163–176). Johannesburg, The Southern African Institute of Mining and Metallurgy.
Tuominen, J., & Kojo, I. V. (2005). Blister flash smelting—Efficient and flexible low-cost continuous copper process. In TMS annual meeting 2005 (San Francisco, CA, USA, February 13–17, 2005).
Winter, C.-J. (2009). Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of change. International Journal of Hydrogen Energy, 34, S1–S52.
Acknowledgments
Support from Outotec and Tekes (Finnish Funding Agency for Technology and Innovation) within the framework of IEA HIA Task 25 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Readers should send their comments on this paper to BhaskarNath@aol.com within 3 months of publication of this issue.
Rights and permissions
About this article
Cite this article
Lokkiluoto, A., Taskinen, P.A., Gasik, M. et al. Novel process concept for the production of H2 and H2SO4 by SO2-depolarized electrolysis. Environ Dev Sustain 14, 529–540 (2012). https://doi.org/10.1007/s10668-012-9342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-012-9342-z