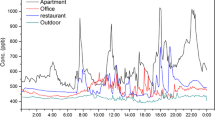
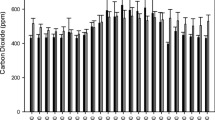
Abstract
Workplaces using office equipment often experience poor air quality in their indoor microenvironments. Office equipment, e.g., photocopier and printer, emits higher levels of ozone (O3) and fine particles. This study uses field measurements to recognize and examine the patterns of the concentrations of O3 and fine particles of aerodynamic diameter less than 1 μ (PM1) in the workplace microenvironment during working and nonworking hours. The O3 and PM1 concentrations were measured simultaneously along with the climate variables from July 2011 to March 2012. The transient levels of these pollutants were also estimated to investigate the observed patterns under normal and closed ventilation conditions. Based on the old and new equipment and their use, different impact scenarios have been developed to study the relationships among O3, PM1, and climate variables. Results revealed that photocopiers contribute to O3, and the printing operations contribute to both O3 and PM1. Further, O3 contributes to PM1 in favorable climatic condition.









Similar content being viewed by others
Notes
The relative effects represent the change in the mean values of the observed parameters such as O3, PM1, T, and RH in percentage for scenario II relative to scenario I and for case I and case II relative to scenario II.
\( \mathrm{S}\mathrm{E}\;\mathrm{of}\;\mathrm{regression}=\sqrt{\left[\frac{1}{\left(n-p\right)}\cdot {\displaystyle \sum_{i=1}^n{\left({\overline{Y}}_i-{Y}_i\right)}^2}\right]} \), where p is the number of independent variables.
Abbreviations
- C t [ppm] :
-
Indoor concentration of CO2
- C ext [ppm] :
-
Outdoor concentration of CO2
- C 0 [ppm] :
-
Indoor concentration of CO2 at time 0
- q CO2 [m 3 /h] :
-
Volumetric indoor emission rate of CO2
- Q [m 3 /h] :
-
Volume flow rate of air entering the space (ventilation rate)
- V [m 3 ] :
-
Volume of indoor space
- t [h] :
-
Time
- V i [m 3 ] :
-
Volume of zone i
- C i [mg/m 3 ] :
-
Pollutant concentration in zone i
- n R :
-
Number of indoor sources in zone i
- R j [mg/h] :
-
Emission rate for j indoor source in zone i
- p :
-
Penetration factor for zone i
- n z :
-
Number of air zones
- Q ki [m 3 /h] :
-
Airflow rate from zone k to zone i and k ≠ i
- C k [mg/m 3 ] :
-
Pollutant concentration in zone k
- Q ik [m 3 /h] :
-
Airflow rate from zone i to zone k and k ≠ i
- n s :
-
Number of interior surface types
- S L [m 2 ] :
-
Surface area for interior surface L in zone i
- k aL :
-
Adsorption rate constant for surface L whose unit depends on f(C i )
- f(C i ) :
-
Function of C i
- k dL :
-
Desorption rate constant for surface L whose unit depends on f(M L )
- f(M L ) :
-
Function of M L
- M L [mg/m 2 ] :
-
Amount of pollutant adsorbed on surface L
- n f :
-
Number of air filters/cleaners associated with zone i
- R p :
-
Pollutant removal efficiency for air filter/cleaner p (dimensionless)
- Q p [m 3 /h] :
-
Airflow rate passing through air filter/cleaner p
- n X :
-
Number of gas phase chemical reactions
- X q [mg/(m 3 .h)] :
-
Rate of chemical reaction q
- S [m 2 ] :
-
Surface area
- K a [m/h] :
-
Adsorption rate constant (deposition velocity)
- C [μg/m 3 or mg/m 3 ] :
-
Concentration in indoor air
- k d [1/h] :
-
Desorption rate constant
- M [mg/m 2 ] :
-
Amount of chemical adsorbed by the surface
- C in,t [mg/m 3 ] :
-
Peak concentrations in the chamber
- C in,o [mg/m 3 ] :
-
Initial background concentrations in the chamber
- C in [mg/m 3 ] :
-
Average value of concentration between the initial background and peak
- λ [1/h] :
-
Total removal rate
- Q s [mg/h] :
-
Emission rate
- ∆t [h] :
-
Time difference between initial background and peak concentration
- Q oi [m 3 /h] :
-
Airflow rate from outside to zone i
- C 0 [μg/m 3 ] :
-
Pollutant concentration in ambient air
- f ji :
-
Filter removal efficiency for airflow from zone j to zone i and j ≠ i
- Q ji [m 3 /h] :
-
Airflow rate from zone j to zone i and j ≠ i
- C j [μg/m 3 ] :
-
Concentration in zone j
- Q ij [m 3 /h] :
-
Airflow rate from zone i to zone j and j ≠ i
- R k [μg/h] :
-
Emission rate for indoor source k
- k D [1/h] :
-
First-order deposition rate constant
- Q 1 [m 3 /h] :
-
Airflow rate passing through the free-standing air filter 1
- F :
-
Removal efficiency for the free-standing air filter 1 (dimensionless)
- n :
-
Number of air zones
- m :
-
Number of indoor sources in zone i
- q :
-
Number of free-standing air filter/cleaners in zone i
- R [μg/h] :
-
Deposition rate
- D vi [m/h] :
-
Deposition velocity for surface i
- S i [m 2 ] :
-
Area of surface i
- N :
-
Number of surface materials
- D k [1/h] :
-
First-order deposition rate constant
References
Lee, S. C., Lam, S., & Fai, H. K. (2001). Characterization of VOC’s, O3 and PM10 emissions from office equipment in an environmental chamber. Building and Environment, 36, 837–842.
Kagi, N., Fujii, S., Horiba, Y., Namiki, N., Ohtani, Y., Emi, H., Tamura, H., & Kim, Y. K. (2007). Indoor air quality for chemical and ultrafine particle contaminants from printers. Building and Environment, 42, 1949–1954.
Wallace, L., Williams, R., Rea, A., & Croghan, C. (2006). Continuous weeklong measurements of personal exposures and indoor concentrations of fine particles for 37health-impaired North Carolina residents for up to four seasons. Atmospheric Environment, 40, 399–414.
Stephens, B., Gall, E. T., & Siegel, J. A. (2011). Measuring the penetration of ambient ozone into residential buildings. Environmental Science & Technology. doi:10.1021/es2028795.
Lee, C. W., & Hsu, D. J. (2007). Measurements of fine and ultrafine particles formation in photocopy centres in Taiwan. Atmospheric Environment, 41, 6598–6609.
Weschler, C. J. (2000). Ozone in indoor environments: concentration and chemistry. Indoor Air, 10, 269–288.
Chan, A. (2002). Indoor and outdoor relationship of particulate matter and nitrogen oxides under different outdoor meteorological conditions. Atmospheric Environment, 36, 1543–1551.
EL-Shobokshy, M. S., & Hussein, F. M. (1967). Correlation between indoor-outdoor inhalable particulate concentration and meteorological variables. Atmospheric Environment, 22(12)1988, 2667–2675.
Chen, G. H., Song, G. X., Jiang, L. L., Zhang, Y. H., Zhang, N. Q., Chen, B. H., & Kan, H. D. (2007). Interaction between ambient particles and ozone and its effect on daily mortality. Biomedical and Environmental Sciences, 20, 502–505.
Destaillats, H., Lunden, M. M., Singer, B. C., Coleman, B. K., Hodgson, A. T., Weschler, C. J., & Nazaroff, W. W. (2000). Indoor secondary pollutants from household product emissions in the presence of ozone: a bench-scale chamber study. Environmental Science and Technology, 40, 4421–4428.
Langer, S., Moldanova, J., Arrhenius, K., Ljungstrom, E., & Ekberg, L. (2008). Ultrafine particles produced by ozone/limonene reactions in indoor air under low/closed ventilation conditions, Sweden. Atmospheric Environment, 42, 4149–4159.
Rai, A. C., Guo, B., Chao-Hsin, L., Zhang, J., Pei, J., & Chen, Q. (2013). Ozone reaction with clothing and its initiated particle generation in an environmental chamber. Atmospheric Environment, 77, 885–892.
Wolkoff, P. (2013). Indoor air pollutants in office environments: assessment of comfort, health, and performance. International Journal of Hygiene and Environmental Health, 216, 371–394.
Carslaw, N., Langer, S., & Wolkoff, P. (2009). New directions: where is the link between reactive indoor chemistry and health effects? Atmospheric Environment, 43, 3808–3809.
Uhde, E., & Salthammer, T. (2007). Impacts of reaction products from building materials and furnishings on indoor air quality—a review of recent advances in indoor chemistry. Atmospheric Environment, 41, 3111–3128.
Sarvar, G., & Corsi, R. (2007). The effects of indoor ozone/limonene reactions on indoor secondary organic aerosols. Atmospheric Environment, 41, 959–973.
Singer, B. C., Coleman, B. K., Destaillats, H., Hodgson, A. T., Lunden, M. M., Weschler, C. J., & Nazaroff, W. W. (2006). Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmospheric Environment, 40, 6696–6710.
Wolkoff, P., Wilkins, C. K., Clausen, P. A., & Neilsen, G. D. (2006). Organic compounds in office environment—sensory irritation, odour, measurements, and the role of reactive chemistry. Indoor Air, 16, 7–19.
Weschler, C. J., & Shield, H. C. (1999). Indoor ozone/terpene reactions as a source of indoor particles. Atmospheric Environment, 33, 2301–2312.
Griffiths, M., & Eftekhari, M. (2008). Control of CO2 in a naturally ventilated classroom. Energy and Buildings, 40, 556–560.
Guo, Z. (1996). Z-30 Indoor Air Quality Simulator. Proceedings of the 7th International Conference on Indoor Air Quality and Climate, vol. 2, 1063–1068.
Knoontz, M., & Nagda, N. (1991). A multi-chamber model for assessing consumer inhalation exposure. Indoor Air, 1, 593–605.
Shair, F. H., & Heitner, K. L. (1974). Theoretical model for relating indoor pollutant concentrations to those outside. Environmental Science & Technology, 8, 444–451.
Grontoft, T., & Raychaudhuri, M. R. (2004). Compilation of tables of surface deposition velocities for O3, NO2 and SO2 to a range of indoor surfaces. Atmospheric Environment, 38(4), 533–544.
Nazaroff, W. W., & Cass, G. R. (1986). Mathematical modelling of chemically reactive pollutants in indoor air. Environmental Science & Technology, 20, 924–934.
Nazaroff, W. W., & Cass, G. R. (1989). Mathematical modelling of indoor aerosol dynamics. Environmental Science & Technology, 23, 157–166.
EPA (2000). Simulation tool kit for indoor air quality and inhalation exposure (IAQX) version 1.0, User’s Guide. EPA-600/R-00-094.
Chen, C., Zhao, B., Wanting, Z., Xinyi, J., & Zhongchao, T. (2012). A methodology for predicting particle penetration factor through cracks of windows and doors for actual engineering application. Building and Environment, 47, 339–348.
Congrong, H.E., Morawska, L., & Taplin, L. (2007). Particle emission characteristics of office printers. International Laboratory for Air Quality and Health, Queensland University of Technology, Brisbane, QLD 4001 Australia. Queensland Department of Public Works Brisbane, QLD 4001, Australia.
Quansah, E., Amekudzi, L. K., & Preko, K. (2012). The influence of temperature and relative humidity on indoor ozone concentrations during the Harmattan. Journal of Emerging Trends in Engineering and Applied Sciences, 3(5), 863–867.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durga Ch., S., Gokhale, S. Monitoring and Assessment of O3 and PM1 in the Microenvironment of a Workplace. Environ Model Assess 20, 521–534 (2015). https://doi.org/10.1007/s10666-014-9440-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10666-014-9440-4