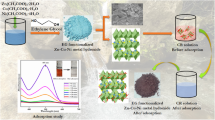
Abstract
Nanoparticles of zinc ferrite (ZnFe2O4) and copper ferrite (CuFe2O4) were synthesized, and characterized, and these materials were applied for removal of organic dyes of alizarin yellow R (AYR), thiazole yellow G (TYG), Congo red (CR), and methyl orange (MO) from industrial wastewater through adsorption technique. Synthesis of ZnFe2O4 and CuFe2O4 was achieved through chemical co-precipitation method. These nanomaterials were characterized for physicochemical properties using XRD, FTIR, BET, VSM, DLS, Zeta-potential, and FESEM-EDX analytical instruments. BET surface areas of ZnFe2O4 and CuFe2O4 were 85.88 m2/g and 41.81 m2/g, respectively. Adsorption-influencing parameters including effect of solution pH, adsorbent quantity, initial concentration of dye pollutant, and contact time were examined. Acidic medium of the solution favored higher percentage of removal of dyes in wastewater. Out of different isotherms, Langmuir equilibrium isotherm showed the best fit with experimental data, indicating monolayer adsorption in the treatment process. The maximum monolayer adsorption capacities were found as 54.58, 37.01, 29.81, and 26.83 mg/g with ZnFe2O4, and 46.38, 30.06, 21.94, and 20.83 mg/g with CuFe2O4 for AYR, TYG, CR, and MO dyes, respectively. From kinetics analysis of the results, it was inferred that pseudo-second-order kinetics were fitting well with better values of coefficient of determination (R2). The removal of four organic dyes from wastewater through adsorption technique using nanoparticles of ZnFe2O4 and CuFe2O4 was observed to be spontaneous and exothermic. From this experimental investigation, it has been inferred that magnetically separable ZnFe2O4 and CuFe2O4 could be a viable option in removal of organic dyes from industrial wastewater.













Similar content being viewed by others
Data availability
The relevant data are available from the authors through a reasonable approach.
References
Ahmad, M. A., Puad, N. A. A., & Bello, O. S. (2014). Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resources and Industry, 6, 18–35. https://doi.org/10.1016/j.wri.2014.06.002
Alam, S., Khan, M. S., Umar, A., Khattak, R., Rahman, N. U., Zekker, I., & Zahoor, M. (2021). Preparation of Pd–Ni nanoparticles supported on activated carbon for efficient removal of basic blue 3 from water. Water, 13(9), 1211. https://doi.org/10.3390/w13091211
Alderete, B. L., da Silva, J., Godoi, R., da Silva, F. R., Taffarel, S. R., da Silva, L. P. et al. (2021). Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after advanced oxidation process treatment. Chemosphere, 263, 128291. https://doi.org/10.1016/j.chemosphere.2020.128291
Al-Tohamy, R., Sun, J., Fareed, M. F., Kenawy, E. R., & Ali, S. S. (2020). Ecofriendly biodegradation of Reactive Black 5 by newly isolated Sterigmatomyces halophilus SSA1575, valued for textile azo dye wastewater processing and detoxification. Scientific Reports, 10(1), 1–16. https://doi.org/10.1038/s41598-020-69304-4
Amir, M., Gungunes, H., Slimani, Y., Tashkandi, N., El Sayed, H. S., Aldakheel, F., & Baykal, A. (2019). Mössbauer studies and magnetic properties of cubic CuFe2O4 nanoparticles. Journal of Superconductivity and Novel Magnetism, 32, 557–564. https://doi.org/10.1007/s10948-018-4733-5
Andhare, D. D., Patade, S. R., Kounsalye, J. S., & Jadhav, K. M. (2020). Effect of Zn doping on structural, magnetic and optical properties of cobalt ferrite nanoparticles synthesized via co-precipitation method. Physica B: Condensed Matter, 583, 412051. https://doi.org/10.1016/j.physb.2020.412051
Ansari, M., Bigham, A., & Ahangar, H. A. (2019). Super-paramagnetic nanostructured CuZnMg mixed spinel ferrite for bone tissue regeneration. Materials Science and Engineering: C, 105, 110084. https://doi.org/10.1016/j.msec.2019.110084
Asbollah, M. A., Sahid, M. S. M., Shahrin, E. W. E., Narudin, N. A. H., Kusrini, E., el Shahri, N. N. M., & al. (2022). Dynamics and thermodynamics for competitive adsorptive removal of methylene blue and rhodamine B from binary aqueous solution onto durian rind. Environmental Monitoring and Assessment, 194(9), 1–14. https://doi.org/10.1007/s10661-022-10332-0
Astuti, W., Chafidz, A., Wahyuni, E. T., Prasetya, A., Bendiyasa, I. M., & Abasaeed, A. E. (2019). Methyl violet dye removal using coal fly ash (CFA) as a dual sites adsorbent. Journal of Environmental Chemical Engineering, 7(5), 103262. https://doi.org/10.1016/j.jece.2019.103262
Attia, A. A., Rashwan, W. E., & Khedr, S. A. (2006). Capacity of activated carbon in the removal of acid dyes subsequent to its thermal treatment. Dyes and Pigments, 69(3), 128–136. https://doi.org/10.1016/j.dyepig.2004.07.009
Atun, G., Ayar, N., Kurtoğlu, A. E., & Ortaboy, S. (2019). A comparison of sorptive removal of anthraquinone and azo dyes using fly ash from single and binary solutions. Journal of Hazardous Materials, 371, 94–107. https://doi.org/10.1016/j.jhazmat.2019.03.006
Azha, S. F., Sellaoui, L., Yunus, E. H. E., Yee, C. J., Bonilla-Petriciolet, A., Lamine, A. B., & Ismail, S. (2019). Iron-modified composite adsorbent coating for azo dye removal and its regeneration by photo-Fenton process: Synthesis, characterization and adsorption mechanism interpretation. Chemical Engineering Journal, 361, 31–40. https://doi.org/10.1016/j.cej.2018.12.050
Badran, I., & Khalaf, R. (2020). Adsorptive removal of alizarin dye from wastewater using maghemite nanoadsorbents. Separation Science and Technology, 55(14), 2433–2448. https://doi.org/10.1080/01496395.2019.1634731
Balakrishnan, V. K., Shirin, S., Aman, A. M., de Solla, S. R., Mathieu-Denoncourt, J., & Langlois, V. S. (2016). Genotoxic and carcinogenic products arising from reductive transformations of the azo dye, Disperse Yellow 7. Chemosphere, 146, 206–215. https://doi.org/10.1016/j.chemosphere.2015.11.119
Cazetta, A. L., Vargas, A. M., Nogami, E. M., Kunita, M. H., Guilherme, M. R., Martins, A. C., et al. (2011). NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chemical Engineering Journal, 174(1), 117–125. https://doi.org/10.1016/j.cej.2011.08.058
Changmai, M., & Purkait, M. K. (2017). Kinetics, equilibrium and thermodynamic study of phenol adsorption using NiFe2O4 nanoparticles aggregated on PAC. Journal of Water Process Engineering, 16, 90–97. https://doi.org/10.1016/j.jwpe.2016.12.011
Cimitan, S., Albonetti, S., Forni, L., Peri, F., & Lazzari, D. (2009). Solvothermal synthesis and properties control of doped ZnO nanoparticles. Journal of Colloid and Interface Science, 329(1), 73–80. https://doi.org/10.1016/j.jcis.2008.09.060
Dhal, J. P., Mishra, B. G., & Hota, G. (2015). Ferrous oxalate, maghemite and hematite nanorods as efficient adsorbents for decontamination of Congo red dye from aqueous system. International Journal of Environmental Science and Technology, 12(6), 1845–1856. https://doi.org/10.1007/s13762-014-0535-x
Ding, Y., Yang, Y., & Shao, H. (2011). High capacity ZnFe2O4 anode material for lithium ion batteries. Electrochimica Acta, 56(25), 9433–9438. https://doi.org/10.1016/j.electacta.2011.08.031
Ding, Z., Wang, W., Zhang, Y., Li, F., & Liu, J. P. (2015). Synthesis, characterization and adsorption capability for Congo red of CoFe2O4 ferrite nanoparticles. Journal of Alloys and Compounds, 640, 362–370. https://doi.org/10.1016/j.jallcom.2015.04.020
El-Naggar, N. E. A., Hussein, M. H., & El-Sawah, A. A. (2018). Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Scientific Reports, 8(1), 1–20. https://doi.org/10.1038/s41598-018-27276-6
Falak, P., Hassanzadeh-Tabrizi, S. A., & Saffar-Teluri, A. (2017). Synthesis, characterization, and magnetic properties of ZnO-ZnFe2O4 nanoparticles with high photocatalytic activity. Journal of Magnetism and Magnetic Materials, 441, 98–104. https://doi.org/10.1016/j.jmmm.2017.05.044
Fan, J., Zhao, Z., Liu, W., Xue, Y., & Yin, S. (2016). Solvothermal synthesis of different phase N-TiO2 and their kinetics, isotherm and thermodynamic studies on the adsorption of methyl orange. Journal of Colloid and Interface Science, 470, 229–236. https://doi.org/10.1016/j.jcis.2016.02.045
Farahmandjou, M., & Soflaee, F. (2015). Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Physical Chemistry Research, 3(3), 191–196. https://doi.org/10.22036/PCR.2015.9193
Franca, A. S., Oliveira, L. S., & Ferreira, M. E. (2009). Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination, 249(1), 267–272. https://doi.org/10.1016/j.desal.2008.11.017
Freitas, N. S., Alzamora, M., Sánchez, D. R., Licea, Y. E., Senra, J. D., & Carvalho, N. M. (2021). Green palladium nanoparticles prepared with glycerol and supported on maghemite for dye removal application. Journal of Environmental Chemical Engineering, 9(1), 104856. https://doi.org/10.1016/j.jece.2020.104856
Gul, K., Sohni, S., Waqar, M., et al. (2016). Functionalization of magnetic chitosan with graphene oxide for removal of cationic and anionic dyes from aqueous solution. Carbohydrate Polymers, 152, 520–531. https://doi.org/10.1016/j.carbpol.2016.06.045
Habibi, M. K., Rafiaei, S. M., Alhaji, A., & Zare, M. (2022). Synthesis of ZnFe2O4: 1 wt% Ce3+/carbon fibers composite and investigation of its adsorption characteristic to remove Congo red dye from aqueous solutions. Journal of Alloys and Compounds, 890, 161901. https://doi.org/10.1016/j.jallcom.2021.161901
Hernández, P., Santiago-Cuevas, A., Palacios-Cabrera, C., Thangarasu, P., Narayanan, J., Kaur, H., & Sharma, A. (2022). Development and applications of Ru and Ce based iron oxides as photocatalysts. Materials Letters, 313, 131720. https://doi.org/10.1016/j.matlet.2022.131720
Hu, X., & Dong, S. (2008). Metal nanomaterials and carbon nanotubes—synthesis, functionalization and potential applications towards electrochemistry. Journal of Materials Chemistry, 18(12), 1279–1295. https://doi.org/10.1039/B713255G
Huang, X., Qin, Y., Ma, Y., & Chen, Y. (2019). Preparation and electromagnetic properties of nanosized ZnFe2O4 with various shapes. Ceramics International, 45(15), 18389–18397. https://doi.org/10.1016/j.ceramint.2019.06.054
Joshi, S., Kumar, M., Chhoker, S., Srivastava, G., Jewariya, M., & Singh, V. N. (2014). Structural, magnetic, dielectric and optical properties of nickel ferrite nanoparticles synthesized by co-precipitation method. Journal of Molecular Structure, 1076, 55–62. https://doi.org/10.1016/j.molstruc.2014.07.048
Kakaei, H., Beygzadeh, M., Golbabaei, F., Ganjali, M. R., Jahangiri, M., & Shahtaheri, S. J. (2019). Preparation of a sepiolite/Cu-BDC nanocomposite and its application as an adsorbent in respirator cartridges for H2S removal. New Journal of Chemistry, 43(29), 11575–11584. https://doi.org/10.1039/C9NJ01623F
Kerroum, M. A., Essyed, A., Iacovita, C., Baaziz, W., Ihiawakrim, D., Mounkachi, O., et al. (2019). The effect of basic pH on the elaboration of ZnFe2O4 nanoparticles by co-precipitation method: structural, magnetic and hyperthermia characterization. Journal of Magnetism and Magnetic Materials, 478, 239–246. https://doi.org/10.1016/j.jmmm.2019.01.081
Khoso, W. A., Haleem, N., Baig, M. A., & Jamal, Y. (2021). Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Scientific Reports, 11(1), 1–10. https://doi.org/10.1038/s41598-021-83363-1
Kim, S. H., & Choi, P. P. (2017). Enhanced Congo red dye removal from aqueous solutions using iron nanoparticles: Adsorption, kinetics, and equilibrium studies. Dalton Transactions, 46(44), 15470–15479. https://doi.org/10.1039/C7DT02076G
Kopcewicz, M., Grabias, A., Kuryliszyn-Kudelska, I., & Dobrowolski, W. (2019). Mössbauer effect study of superparamagnetic behavior of ZnFe2O4 nanoparticles formed in ZnO doped with Fe2O3. Physica Status Solidi (b), 256(5), 1800223. https://doi.org/10.1002/pssb.201800223
Kumar, R., Ansari, S. A., Barakat, M. A., Aljaafari, A., & Cho, M. H. (2018). A polyaniline@ MoS 2-based organic–inorganic nanohybrid for the removal of Congo red: Adsorption kinetic, thermodynamic and isotherm studies. New Journal of Chemistry, 42(23), 18802–18809. https://doi.org/10.1039/C8NJ02803F
Kumar, R., Ansari, M. O., Parveen, N., Barakat, M. A., & Cho, M. H. (2015). Simple route for the generation of differently functionalized PVC@ graphene–polyaniline fiber bundles for the removal of Congo red from wastewater. RSC Advances, 5(76), 61486–61494. https://doi.org/10.1039/C5RA10378A
Kusic, H., Juretic, D., Koprivanac, N., Marin, V., & Božić, A. L. (2011). Photooxidation processes for an azo dye in aqueous media: Modeling of degradation kinetic and ecological parameters evaluation. Journal of Hazardous Materials, 185(2–3), 1558–1568. https://doi.org/10.1016/j.jhazmat.2010.10.087
Li, J., Ng, D. H., Song, P., Song, Y., & Kong, C. (2015). Bio-inspired synthesis and characterization of mesoporous ZnFe2O4 hollow fibers with enhancement of adsorption capacity for acid dye. Journal of Industrial and Engineering Chemistry, 23, 290–298. https://doi.org/10.1016/j.jiec.2014.08.031
Li, W., Mu, B., & Yang, Y. (2019). Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresource Technology, 277, 157–170. https://doi.org/10.1016/j.biortech.2019.01.002
Lima, É. C., Adebayo, M. A., & Machado, F. M. (2015). Kinetic and equilibrium models of adsorption. Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications, 33–69. https://doi.org/10.1007/978-3-319-18875-1_3
Lokhande, P. E., Kulkarni, S., Chakrabarti, S., Pathan, H. M., Sindhu, M., Kumar, D., & Tiwari, A. (2022). The progress and roadmap of metal–organic frameworks for high-performance supercapacitors. Coordination Chemistry Reviews, 473, 214771. https://doi.org/10.1016/j.ccr.2022.214771
Malik, A. Q., Amin, O., Sathish, M., & Kumar, D. (2022). Synthesis, characterization, photocatalytic effect of CuS-ZnO nanocomposite on photodegradation of Congo red and phenol pollutant. Inorganic Chemistry Communications, 143, 109797. https://doi.org/10.1016/j.inoche.2022.109797
Margabandhu, M., Sendhilnathan, S., Senthilkumar, S., & Gajalakshmi, D. (2017). Investigation of structural, morphological, magnetic properties and biomedical applications of Cu2+ substituted uncoated cobalt ferrite nanoparticles. Brazilian Archives of Biology and Technology, 59. https://doi.org/10.1590/1678-4324-2016161046
Mukherjee, S., Kumar, S., Misra, A. K., & Fan, M. (2007). Removal of phenols from water environment by activated carbon, bagasse ash and wood charcoal. Chemical Engineering Journal, 129(1–3), 133–142. https://doi.org/10.1016/j.cej.2006.10.030
Naresh, U., Kumar, R. J., & Naidu, K. C. B. (2019). Hydrothermal synthesis of barium copper ferrite nanoparticles: nanofiber formation, optical, and magnetic properties. Materials Chemistry and Physics, 236, 121807. https://doi.org/10.1016/j.matchemphys.2019.121807
Niederberger, M. (2007). Nonaqueous sol–gel routes to metal oxide nanoparticles. Accounts of Chemical Research, 40(9), 793–800. https://doi.org/10.1021/ar600035e
Noreen, S., Mustafa, G., Ibrahim, S. M., Naz, S., Iqbal, M., Yaseen, M., et al. (2020). Iron oxide (Fe2O3) prepared via green route and adsorption efficiency evaluation for an anionic dye: kinetics, isotherms and thermodynamics studies. Journal of Materials Research and Technology, 9(3), 4206–4217. https://doi.org/10.1016/j.jmrt.2020.02.047
Nouren, S., Bhatti, H. N., Iqbal, M., Bibi, I., Kamal, S., Sadaf, S., et al. (2017). By-product identification and phytotoxicity of biodegraded Direct Yellow 4 dye. Chemosphere, 169, 474–484. https://doi.org/10.1016/j.chemosphere.2016.11.080
Parisi, F., Lazzara, G., Merli, M., Milioto, S., Princivalle, F., & Sciascia, L. (2019). Simultaneous removal and recovery of metal ions and dyes from wastewater through montmorillonite clay mineral. Nanomaterials, 9(12), 1699. https://doi.org/10.3390/nano9121699
Pietrzyk, P., Phuong, N. T., Olusegun, S. J., Hong Nam, N., Thanh, D. T. M., Giersig, M., & Osial, M. (2022). Titan Yellow and Congo Red removal with superparamagnetic iron-oxide-based nanoparticles doped with zinc. Magnetochemistry, 8(8), 91. https://doi.org/10.3390/magnetochemistry8080091
Rachna, K., Agarwal, A., & Singh, N. B. (2018). Preparation and characterization of zinc ferrite—Polyaniline nanocomposite for removal of rhodamine B dye from aqueous solution. Environmental Nanotechnology, Monitoring & Management, 9, 154–163. https://doi.org/10.1016/j.enmm.2018.03.001
Raju, G., Murali, N., Prasad, M. S. N. A., Suresh, B., Babu, D. A., Kiran, M. G., et al. (2019). Effect of chromium substitution on the structural and magnetic properties of cobalt ferrite. Materials Science for Energy Technologies, 2(1), 78–82. https://doi.org/10.1016/j.mset.2018.11.001
Rao, W., Piliouras, P., Wang, X., Guido, A., Kugler, K., Sieren, B., et al. (2020). Zwitterionic dye rhodamine B (RhB) uptake on different types of clay minerals. Applied Clay Science, 197, 105790. https://doi.org/10.1016/j.clay.2020.105790
Rashad, M. M., Mohamed, R. M., Ibrahim, M. A., Ismail, L. F. M., & Abdel-Aal, E. A. (2012). Magnetic and catalytic properties of cubic copper ferrite nanopowders synthesized from secondary resources. Advanced Powder Technology, 23(3), 315–323. https://doi.org/10.1016/j.apt.2011.04.005
Salman, M., Athar, M., Shafique, U., & Din, M. I. (2011). Adsorption modeling of alizarin yellow on untreated and treated charcoal. Turkish Journal of Engineering and Environmental Sciences, 35(3), 389–396.
Sathishkumar, K., AlSalhi, M. S., Sanganyado, E., Devanesan, S., Arulprakash, A., & Rajasekar, A. (2019). Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. Journal of Photochemistry and Photobiology B: Biology, 200, 111655. https://doi.org/10.1016/j.jphotobiol.2019.111655
Shang, Y., Li, X., Yang, Y., Wang, N., Zhuang, X., & Zhou, Z. (2020). Optimized photocatalytic regeneration of adsorption-photocatalysis bifunctional composite saturated with Methyl Orange. Journal of Environmental Sciences, 94, 40–51. https://doi.org/10.1016/j.jes.2020.03.044
Singh, J., Kumar, D., Kumar, P. S., Aguilar, C. H., Vo, D. V., Sharma, A., & Kaur, H. (2021). Magnetically active Ag–Zn nanoferrites synthesized by solution combustion route: physical chemical studies and density functional theory analysis. Materials Today Chemistry, 22, 100588. https://doi.org/10.1016/j.mtchem.2021.100588
Singh, J., Sharma, S., Soni, S., Sharma, S., & Singh, R. C. (2019). Influence of different milling media on structural, morphological and optical properties of the ZnO nanoparticles synthesized by ball milling process. Materials Science in Semiconductor Processing, 98, 29–38. https://doi.org/10.1016/j.mssp.2019.03.026
Steter, J. R., Barros, W. R., Lanza, M. R., & Motheo, A. J. (2014). Electrochemical and sonoelectrochemical processes applied to amaranth dye degradation. Chemosphere, 117, 200–207. https://doi.org/10.1016/j.chemosphere.2014.06.085
Tadjarodi, A., Imani, M., & Salehi, M. (2015). ZnFe2O4 nanoparticles and a clay encapsulated ZnFe2O4 nanocomposite: Synthesis strategy, structural characteristics and the adsorption of dye pollutants in water. RSC Advances, 5(69), 56145–56156. https://doi.org/10.1039/C5RA02163D
Tkaczyk, A., Mitrowska, K., & Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Science of The Total Environment, 717, 137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Uddin, M. K., & Baig, U. (2019). Synthesis of Co3O4 nanoparticles and their performance towards methyl orange dye removal: Characterisation, adsorption and response surface methodology. Journal of Cleaner Production, 211, 1141–1153. https://doi.org/10.1016/j.jclepro.2018.11.232
Vergis, B. R., Hari Krishna, R., Kottam, N., Nagabhushana, B. M., Sharath, R., & Darukaprasad, B. (2018). Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. Journal of Nanostructure in Chemistry, 8(1), 1–12. https://doi.org/10.1007/s40097-017-0249-y
Wang, L., & Wang, A. (2007). Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. Journal of Hazardous Materials, 147(3), 979–985. https://doi.org/10.1016/j.jhazmat.2007.01.145
Wang, S., & Li, H. (2005). Dye adsorption on unburned carbon: Kinetics and equilibrium. Journal of Hazardous Materials, 126(1–3), 71–77. https://doi.org/10.1016/j.jhazmat.2005.05.049
Wu, Z., Joo, H., & Lee, K. (2005). Kinetics and thermodynamics of the organic dye adsorption on the mesoporous hybrid xerogel. Chemical Engineering Journal, 112(1–3), 227–236. https://doi.org/10.1016/j.cej.2005.07.011
Xiao, Y., & Hill, J. M. (2017). Impact of pore size on fenton oxidation of methyl orange adsorbed on magnetic carbon materials: Trade-off between capacity and regenerability. Environmental Science & Technology, 51(8), 4567–4575. https://doi.org/10.1021/acs.est.7b00089
Xu, H., Zhang, Y., Cheng, Y., Tian, W., Zhao, Z., & Tang, J. (2019). Polyaniline/attapulgite-supported nanoscale zero-valent iron for the rival removal of azo dyes in aqueous solution. Adsorption Science & Technology, 37(3–4), 217–235. https://doi.org/10.1177/0263617418822917
Yang, Y., Jing, L., Yu, X., Yan, D., & Gao, M. (2007). Coating aqueous quantum dots with silica via reverse microemulsion method: Toward size-controllable and robust fluorescent nanoparticles. Chemistry of Materials, 19(17), 4123–4128. https://doi.org/10.1021/cm070798m
Yasui, K., Sasaki, K., Ikeda, N., & Kinoshita, H. (2019). Dye adsorbent materials based on porous ceramics from glass fiber-reinforced plastic and clay. Applied Sciences, 9(8), 1574. https://doi.org/10.3390/app9081574
Yavuz, E., Tokalıoğlu, Ş, & Patat, Ş. (2018). Magnetic dispersive solid phase extraction with graphene/ZnFe2O4 nanocomposite adsorbent for the sensitive determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry. Microchemical Journal, 142, 85–93. https://doi.org/10.1016/j.microc.2018.06.019
Zhang, J., Ji, H., Liu, Z., Zhang, L., Wang, Z., Guan, Y., & Gao, H. (2022). 3D porous structure-inspired lignocellulosic biosorbent of medulla tetrapanacis for efficient adsorption of cationic dyes. Molecules, 27(19), 6228. https://doi.org/10.3390/molecules27196228
Zhang, S., Niu, H., Cai, Y., Zhao, X., & Shi, Y. (2010). Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chemical Engineering Journal, 158(3), 599–607. https://doi.org/10.1016/j.cej.2010.02.013
Zhao, T., Li, P., Tai, C., She, J., Yin, Y., & Zhang, G. (2018). Efficient decolorization of typical azo dyes using low-frequency ultrasound in presence of carbonate and hydrogen peroxide. Journal of Hazardous Materials, 346, 42–51. https://doi.org/10.1016/j.jhazmat.2017.12.009
Zheng, Y., Cheng, B., Fan, J., Yu, J., & Ho, W. (2021). Review on nickel-based adsorption materials for Congo red. Journal of Hazardous Materials, 403, 123559. https://doi.org/10.1016/j.jhazmat.2020.123559
Zolgharnein, J., Asanjrani, N., Bagtash, M., & Azimi, G. (2014). Multi-response optimization using Taguchi design and principle component analysis for removing binary mixture of alizarin red and alizarin yellow from aqueous solution by nano γ-alumina. Spectrochimica Acta Part a: Molecular and Biomolecular Spectroscopy, 126, 291–300. https://doi.org/10.1016/j.saa.2014.01.100
Acknowledgements
We are grateful to Shiv Nadar Institution of Eminence Deemed to be University for the financial support required in conducting this study and for providing XRD, FTIR, DLS, and zeta potential analytical facilities for material characterization. We thank Advance Centre for Material Sciences, IIT Kanpur, for BET analytical facility, and Scientium Analyze Solutions, Jaipur, for VSM and FESEM analytical facilities.
Author information
Authors and Affiliations
Contributions
DP contributed to conceptualization, methodology development, conducting experiments, data analysis and processing, modeling work, and writing the original manuscript. SB contributed to research guidance, frame-working scopes of research, resource generation, and review, correction, and revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, D.J., Behera, S.N. Synthesizing nanoparticles of zinc and copper ferrites and examining their potential to remove various organic dyes through comparative studies of kinetics, isotherms, and thermodynamics. Environ Monit Assess 195, 591 (2023). https://doi.org/10.1007/s10661-023-11177-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11177-x