Abstract
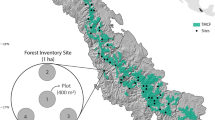
Tropical forests sequester six times higher carbon than that released by humans annually into the atmosphere. These biodiversity-rich tropical forests have high net primary productivity (NPP), which differs among constituent plant communities. Tropical moist deciduous forests occupy 179,335 km2 of India’s geographical area and constitute 44% of the country’s total protected area (PA) forests. The productivity of these forests has neither been estimated specifically nor precisely. We measured the annual NPP of three predominant distinct community types, viz., mixed (DM), sal (SM), and teak (TP), in a tropical moist deciduous forest in northern India. The NPP was estimated from tree biomass data collected from nine long-term ecological research (LTER) plots of 1 ha each representing the above three community types. The estimated annual NPP were 10.28, 6.25, and 9.79 Mg ha−1 year−1 in DM; 8.93, 7.09, and 10.59 Mg ha−1 year−1 in SM; and 14.57, 7.14, and 13.56 Mg ha−1 year−1 in TP for the years 2010, 2011, and 2012, respectively. The NPP was correlated with tree density, height and DBH, species richness, diversity, microclimatic and edaphic variables, and leaf area index (LAI) using principal component analysis (PCA) and generalized linear modeling (GLM). Air temperature and humidity were strongly related to NPP in all the community types, while “complementarity” and “selection effects” contributed to the NPP in both the sal and mixed forest communities with equal importance, and the NPP in teak plantation ould point to “dominance effect.”







Similar content being viewed by others
Data availability
I, Soumit K. Behera, on behalf of all the authors declare that we have given all the required data in the above manuscript. We do not have any additional data to give with the above MS. We will share the raw data if required.
References
Asner, G. P., Scurlock, J. M. O., & Hicke, J. A. (2003). Global synthesis of leaf area index observation: Implication for ecological and remote sensing studies. Global Ecology and Biogeography, 12, 191–205.
Barik, S. K., Pandey, H. N., Tripathi, R. S., & Rao, P. (1992). Microenvironmental variability and species diversity in tree fall gaps in a sub-tropical broadleaved forest. Vegetatio, 103(1), 31–40.
Behera, S. K., Behera, M. D., & Tuli, R. (2015). An indirect method of estimating leaf area index in a tropical deciduous forest of India. Ecological Indicators, 58, 356–364.
Behera, S. K., Mishra, A. K., Sahu, N., Kumar, A., Singh, N., et al. (2012). The study of microclimate in response to different plant community association in tropical moist deciduous forest from northern India. Biodiversity and Conservation, 21, 1159–1176.
Behera, S. K., Sahu, N., Mishra, A. K., Bargali, S. S., Behera, M. D., & Tuli, R. (2017). Aboveground biomass and carbon stock assessment in Indian tropical deciduous forest and relationship with stand structural attributes. Ecological Engineering, 99, 513–524.
Bonan, G. B. (2008). Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science, 320, 1444–1449.
Bonan, G. B., & Shugart, H. H. (1989). Environmental factors and ecological processes in boreal forests. The Annual Review of Ecology, Evolution, and Systematics, 20, 1–28.
Bondeau, A., Kicklighter, D.W., Kaduk, J., and the Participants of the Potsdam NPP Model Intercomparison. (1999). Comparing global models of terrestrial net primary productivity (NPP): Importance of vegetation structure on seasonal NPP estimates. Global Change Biology, 5, 35–45.
Cardinale, B. J., Wright, J. P., Cadotte, M. W., Caroll, I. T., Hector, A., et al. (2007). Impact of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences of the United States of America, 104, 18123–18128.
Champion, H. G., & Seth, S. K. (1968). A revised survey of the forest types of India. Manager of publications.
Chapin, F. S., & Eviner, V. T., III. (2003). Biogeochemistry of terrestrial net primary production. In W. Schlesinger (Ed.), Biogeochemistry: Treatise on geochemistry (pp. 215–247). Elsevier.
Chave, J., Andalo, C., Brown, S., Cairns, M. A., Chambers, J. Q., et al. (2005). Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia, 145, 87–99.
Chen, J. M., Rich, P. M., Gower, S. T., Norman, J. M., & Plummer, S. (1997). Leaf area index of boreal forests: Theory, techniques, and measurements. Journal of Geophysical Research, 102, 29429–29443.
Chen, S., Wang, W., Xu, W., Wang, Y., Wan, H., Chen, D., & Bai, Y. (2018). Plant diversity enhances productivity and soil carbon storage. Proceedings of the National Academy of Sciences, 115(16), 4027–4032.
Chitale, V. S., & Behera, M. D. (2014). Analysing land and vegetation cover dynamics during last three decades in Katerniaghat Wildlife Sanctuary India. Journal of Earth System Science, 123(7), 1467–1479.
Churkina, G., Running, S. W., Schloss, A. L., and the Participants of the Potsdam NPP Model Intercomparison. (1999). Comparing global models of terrestrial net primary productivity (NPP): The importance of water availability. Global Change Biology, 5, 46–55.
Clark, D. A., Brown, S., Kicklighter, D. W., Chambers, J. Q., Thomlinson, J. R., et al. (2001a). Measuring net primary production in forests: Concepts and field methods. Ecological Applications, 11, 356–370.
Clark, D. A., Brown, S., Kicklighter, D. W., Chambers, J. Q., Thomlinson, J. R., et al. (2001b). Net primary production in tropical forests: An evaluation and synthesis of existing field data. Ecological Applications, 11, 371–384.
Das, A. K., & Ramakrishnan, P. S. (1985). Litter dynamics in Khasi pine (Pinus kesiya Royle ex Gordon) of North-eastern India. Forest Ecology and Management, 10, 135–153.
Devi, L. S., & Yadava, P. S. (2009). Aboveground biomass and net primary productivity of semi-evergreen tropical forest of Manipur, north-eastern India. Journal of Forestry Research, 20, 151–155.
David, H. C., de Araújo, E. J. G., Morais, V. A., Scolforo, J. R. S., Marques, J. M., Netto, S. P., & MacFarlane, D. W. (2017). Carbon stock classification for tropical forests in Brazil: Understanding the effect of stand and climate variables. Forest Ecology and Management, 404, 241–250.
Ducey, M. J., Zarin, D. J., Vasconcelos, S. S., & Araújo, M. M. (2009). Biomass equations for forest regrowth in the eastern Amazon using randomized branch sampling. Acta Amazonica, 39, 349–360.
George, M., Varghees, G., & Manivachakam, P. (1990). Nutrient cycling in Indian tropical dry deciduous forest ecosystem. Proceeding of the Seminar on Forest Productivity held at F.R.I. Dehradun, India, 23–24 April 1990, pp. 289–297.
Gherardi, L. A., & Sala, O. E. (2020). Global patterns and climatic controls of belowground net carbon fixation. Proceedings of the National Academy of Sciences, 117(33), 20038–20043.
Girardin, C. A. J., Mahli, Y., Aragao, L. E. O. C., Mamani, M., Huaraca Huasco, W., et al. (2010). Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Global Change Biology, 16, 3176–3192.
Gower, S. T., McMurtrie, R. E., & Murty, D. (1996). Aboveground net primary production decline with stand age: Potential causes. Trends in Ecology and Evolution, 11, 378–382.
Huang, J. G., Ma, Q., Rossi, S., Biondi, F., Deslauriers, A., Fonti, P., & Ziaco, E. (2020). Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proceedings of the National Academy of Sciences, 117(34), 20645–20652.
IGBP, Terrestrial Carbon Working Group. (1998). The terrestrial carbon cycle: Implications for the Kyoto protocol. Science, 280, 1393–1394.
Kooijmans, L. M., Sun, W., Aalto, J., Erkkilä, K. M., Maseyk, K., Seibt, U., & Chen, H. (2019). Influences of light and humidity on carbonyl sulfide-based estimates of photosynthesis. Proceedings of the National Academy of Sciences, 116(7), 2470–2475.
Laurance, W. F., Fearnside, P. M., Laurance, S. G., Delamonica, P., Lovejoy, T. E., et al. (1999). Relationship between soils and Amazon forest biomass: A landscape-scale study. Forest Ecology and Management, 118, 127–138.
Leuschner, C., Moser, G., Bertsch, C., Röderstein, M., & Hertel, D. (2007). Large altitudinal increase in tree root/shoot ratio in tropical mountain forests of Ecuador. Basic and Applied Ecology, 8, 219–230.
Liang, J., Zhou, M., Tobin, P. C., McGuire, A. D., & Reich, P. B. (2015). Biodiversity influences plant productivity through niche–efficiency. Proceedings of the National Academy of Sciences, 112(18), 5738–5743.
Linger, E., Hogan, J. A., Cao, M., Zhang, W. F., Yang, X. F., & Hu, Y. H. (2020). Precipitation influences on the net primary productivity of a tropical seasonal rainforest in Southwest China: A 9-year case study. Forest Ecology and Management, 467, 118153.
Loreau, M., & Hector, A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature, 412, 72–76.
Lu, X., Vitousek, P. M., Mao, Q., Gilliam, F. S., Luo, Y., Turner, B. L., & Mo, J. (2021). Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proceedings of the National Academy of Sciences, 118(16), e2020790118.
Malhi, Y., Doughty, C., & Galbraith, D. (2011). The allocation of ecosystem net primary productivity in tropical forests. Philosophical Transactions of the Royal Society Biological Sciences, 366, 3225–3245.
Malhi, Y., Eduardo, L., Aragao, C., Metcalfe, D. B., Paiva, R., et al. (2005). Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Global Change Biology, 15, 1255–1274.
Malhi, Y., & Grace, J. (2000). Tropical forests and atmospheric carbon dioxide. Trends in Ecology and Evolution, 15, 332–337.
Martinez-Yrizar, A., Sarukhan, J., Perez-Jimenez, A., Rincon, E., Maass, J. M., et al. (1992). Above-ground phytomass of a tropical deciduous forest on the coast of Jalisco, Mexico. Journal of Tropical Ecology, 8, 87–96.
McCarthy, H. R., Oren, R., Finzi, A. C., & Johnsen, K. H. (2006). Canopy leaf area constrains [CO2]-induced enhancement of productivity and partitioning among aboveground carbon pools. Proceedings of the National Academy of Sciences, 103(51), 19356–19361.
Medvigy, D., Wofsy, S. C., Munger, J. W., & Moorcroft, P. R. (2010). Responses of terrestrial ecosystems and carbon budgets to current and future environmental variability. Proceedings of the National Academy of Sciences of the United States of America, 107, 8275–8280.
Misra, R., Singh, J. S., & Singh, K. P. (1967). Preliminary observations on the production of dry matter by sal (Shorea robusta Gaertn. f.). Tropical Ecology, 8, 94–104.
Montagnini, F., & Jordan, C. F. (2005). Tropical forest ecology: The basis for conservation and management. Springer Science & Business Media.
Mooney, H., & Bullock, S. (Eds.). (1994). Tropical deciduous forest ecosystems. Cambridge University Press.
Nogués-Bravo, D. (2009). Predicting the past distribution of species climatic niches. Global Ecology and Biogeography, 18(5), 521–531.
Norby, R. J., DeLucia, E. H., Gielen, B., Calfapietra, C., Giardina, C. P., King, J. S., & Oren, R. (2005). Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings of the National Academy of Sciences, 102(50), 18052–18056.
Paquette, A., & Messier, C. (2011). The effect of biodiversity on tree productivity: From temperate to boreal forests. Global Ecology and Biogeography, 20, 170–180.
Poorter, L., Sande, M. T., Thompson, J., Arets, E. J. M. M., Alarcón, A., et al. (2015). Diversity enhances carbon storage in tropical forests. Global Ecology and Biogeography, 24, 1–15.
Raman, S. S. (1976). Biological productivity of Shorea plantations. Indian Forester, 102, 174–184.
Rana, B. S., Singh, S. P., & Singh, R. P. (1988). Biomass and productivity of central Himalayan Sal (Shorea robusta) forest. Tropical Ecology, 29, 1–5.
Roy, P. S., Behera, M. D., Murthy, M. S. R., Roy, A., Singh, S., Kushwaha, S. P. S., & Ramachandran, R. M. (2015). New vegetation type map of India prepared using satellite remote sensing: Comparison with global vegetation maps and utilities. International Journal of Applied Earth Observation and Geoinformation, 39, 142–159.
Saldarriaga, J. G., West, D. C., Tharp, M. L., & Uhl, C. (1988). Long-term chronosequence of forest succession in the upper Rio Negro of Colombia and Venezuela. Journal of Ecology, 76, 938–958.
Shirima, D. D., Pfeifer, M., Platts, P. J., Totland, Ø., & Moe, S. R. (2015). Interactions between canopy structure and herbaceous biomass along environmental gradients in moist forest and dry Miombo woodland of Tanzania. PLoS ONE, 10, 1–15.
Singh, J. S., Singh, S. P., Saxena, A. K., & Rawat, Y. S. (1984a). The forest vegetation of Silent Valley, India. Tropical Rain Forest. The Leeds symposium. pp. 25–52.
Singh, J. S., Rawat, Y. S., & Chaturvedi, O. P. (1984b). Replacement of oak forest with pine in the Himalaya affects the nitrogen cycle. Nature, 311, 54–56.
Singh, L., & Singh, J. S. (1991). Storage and flux of nutrient in a dry tropical forest in India. Annals of Botany, 68, 275–284.
Slik, J. W. F., Aiba, S., Brearley, F. Q., Cannon, C. H., Forshed, O., et al. (2010). Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo’s tropical forests. Global Ecology and Biogeography, 19, 50–60.
Swamy, S. L., Dutt, C. B. S., Murthy, M. S. R., Mishra, A., & Bargali, S. S. (2010). Floristics and dry matter dynamics of tropical wet evergreen forests of Western Ghats, India. Current Science, 99, 353–364.
Taylor, P. G., Cleveland, C. C., Wieder, W. R., Sullivan, B. W., Doughty, C. E., Dobrowski, S. Z., & Townsend, A. R. (2017). Temperature and rainfall interact to control carbon cycling in tropical forests. Ecology Letters, 20(6), 779–788.
Tian, H., Mellilo, J. M., Kicklighter, D. W., McGuire, A. D., Helfrich III, J. V. K., et al. (1998). Effect of inter annual climate variability on carbon storage in Amazonian ecosystems. Nature, 396, 664–667.
Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M., et al. (1997). The influence of functional diversity and composition on ecosystem processes. Science, 277, 1300–1302.
Toky, O. P., & Ramakrishnan, P. S. (1983). Secondary succession following slash-and-burn agriculture in north-eastern India. I. Biomass, litterfall and productivity. Journal of Ecology, 71, 735–745.
Tripathi, P., Behera, M. D., Behera, S. K., & Sahu, N. (2019). Investigating the contribution of climate variables to estimates of net primary productivity in a tropical deciduous forest in India. Environmental Monitoring and Assessment, 191(3), 1–15.
Vilà, M., Vayreda, J., Comas, L., Ibáñez, J., Mata, T., et al. (2007). Species richness and wood production: A positive association in Mediterranean forests. Ecology Letters, 10, 241–250.
Wang, W., Ichii, K., Hashimoto, H., Michaelis, A. R., Thornton, P. E., et al. (2009). A hierarchical analysis of terrestrial ecosystem model Biome-BGC: Equilibrium analysis and model calibration. Ecological Modelling, 220, 2009–2023.
Whittaker, A. H., & Woodwell, G. M. (1968). Dimension and production relations of trees and shrubs in the Brookhaven forest. New York. Journal of Ecology, 56, 1–25.
Acknowledgements
The director of CSIR-National Botanical Research Institute, Lucknow, India, is sincerely acknowledged for providing the necessary facilities. Dr. Nayan Sahu, Dr. Ashish K. Mishra, Dr. Omesh Bajpai, and Dr. Niraj Singh are also acknowledged for assisting in the field measurements. Thanks are also due to PCCF (Wildlife), Government of Uttar Pradesh, Lucknow, the Field director of Dudhwa National Park, Lakhimpur, and the divisional forest officer at Katarniaghat Wildlife Sanctuary, Bahraich, for granting permission to carry out the field works and extending on-site support. The valuable suggestions given by both the anonymous reviewers and the editor are sincerely acknowledged.
Funding
The funds to carry out this work were received from CSIR, New Delhi, under NWP-020 and BSC-0109. We thank the institutional ethics committee for granting institutional MS no CSIR-NBRI_MS/2022/07/04.
Author information
Authors and Affiliations
Contributions
Soumit K. Behera planned the experiment, collected the field data for calculation of NPP, and measured the different eco-physiological parameters. Mukunda D. Behera and Rakesh Tuli supervised the PhD work of Soumit K. Behera and reviewed the manuscript. Saroj K. Barik performed the statistical data analysis and provided valuable suggestions and reviews for improving the contents of this paper. Soumit K. Behera prepared the first draft of the manuscript. All the authors contributed equally in revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behera, S.K., Behera, M.D., Tuli, R. et al. Atmospheric temperature and humidity demonstrated strong correlation with productivity in tropical moist deciduous forests. Environ Monit Assess 195, 69 (2023). https://doi.org/10.1007/s10661-022-10668-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10668-7