Abstract
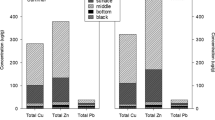
Metal biogeochemistry in the sediment, water, and the sediment–water interface (SWI) was studied in a free water surface constructed wetland. Concentrations of labile copper (Cu), zinc (Zn), sulfate, chloride, and dissolved organic carbon (DOC) were measured with the diffusive gradients in thin film (DGT) and peeper. A good agreement between peeper- and DGT-measured metals was observed for Cu (regression r2 = 0.3, 95% CI of the slopes > 0), but not for Zn (95% CI of the slopes overlapped with 0), which was attributed to the different complexed compounds between Cu and Zn in porewater. The depth profile of labile metals in sediment porewater varied with time and was consistent with the solid-phase metal deposition, showing higher concentrations in the surface layer (3 to − 3 cm) than in the bottom layer (− 4 to − 13 cm). The depth-averaged labile Cu and Zn concentrations measured by DGT were 1.0 and 3.1 µg/L, and labile sulfate, chloride, and DOC concentrations measured by peeper were 1.8, 3.6, and 2.1 mg/L, respectively. A sharp decrease in sulfate occurred in September when sulfate concentrations became the lowest among sampling months. This was caused by the seasonal sulfur cycles in the wetland, where the dominant sulfur reaction is sulfate reduction in warm seasons and sulfide oxidation in cold seasons. Different metal-removal mechanisms were observed in the two wetland cells; sulfur dynamics controlled the removal processes in the cell without frequent disturbance but failed to influence metal removal in the cell with frequent disturbance due to the interruption of anoxic layers. The flux ratios that compare labile element concentrations between the water column and the SWI (R-Cu, R-Zn, R-DOC, R-sulfate, and R-chloride) were generated to determine metal diffusive fluxes at the interface. Labile Zn was mostly transported from the water to the SWI during all seasons (R-Zn < 1 for all months except January). Labile Cu moved from the SWI to the water during the warm months (R-Cu < 1), which was explained by the bioturbation-induced transport of organic matter based on the positive correlations between R-Cu and R-DOC. In general, sediment can serve either as a sink or a source depending on the environmental conditions, metal speciation, and presence of living organisms. Metal flux at the SWI is a key component in the biogeochemical cycling of a constructed wetland.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Altman, D. G. (2000). Confidence intervals in practice. In D. ltman, D. Machin, T. N. Bryant, & M. J. Gardner (Eds.), Statistics with confidence: Confidence intervals and statistical guidelines (pp. 6–15). British Medical Journal Books.
Amato, E. D., Simpson, S. L., Remaili, T. M., Spadaro, D. A., Jarolimek, C. V., & Jolley, D. F. (2016). Assessing the effects of bioturbation on metal bioavailability in contaminated sediments by diffusive gradients in thin films (DGT). Environmental Science & Technology, 50(6), 3055–3064. https://doi.org/10.1021/acs.est.5b04995
Anderson, D. R., Burnham, K. P., & Thompson, W. L. (2000). Null hypothesis testing: Problems, prevalence, and an alternative. J. Wildlife Manage, 64, 912–923. https://doi.org/10.2307/3803199
Baddar, Z. E., Peck, E., & Xu, X. Y. (2021). Temporal deposition of copper and zinc in the sediments of metal removal constructed wetlands. Plos One, 16(8). https://doi.org/10.1371/journal.pone.0255527
Blasco, J., Saenz, V., & Gomez-Parra, A. (2000). Heavy metal fluxes at the sediment-water interface of three coastal ecosystems from south-west of the Iberian Peninsula. Science Total Environment, 247(2–3), 189–199. https://doi.org/10.1016/S0048-9697(99)00490-8
Brown, K., & Caldwell, D. (2016). Sanpling pore water sediments [SOPs](Standard Operating Procedures, Issue.
Buaisha, M., Balku, S., & Ozalp-Yaman, S. (2020). Heavy metal removal investigation in conventional activated sludge systems. Civil Engineering Journal-Tehran, 6(3), 470–477. https://doi.org/10.28991/cej-2020-03091484
Burton, E. D., Phillips, I. R., & Hawker, D. W. (2005). Geochemical partitioning of copper, lead, and zinc in benthic, estuarine sediment profiles. Journal of Environmental Quality, 34(1), 263–273. https://doi.org/10.2134/jeq2005.0263
Carignan, R., Rapin, F., & Tessier, A. (1985). Sediment porewater sampling for metal analysis—A comparison of techniques. Geochimica Et Cosmochimica Acta, 49(11), 2493–2497. https://doi.org/10.1016/0016-7037(85)90248-0
Christensen, T. H., Bjerg, P. L., Banwart, S. A., Jakobsen, R., Heron, G., & Albrechtsen, H. J. (2000). Characterization of redox conditions in groundwater contaminant plumes. Journal of Contaminant Hydrology, 45(3–4), 165–241. https://doi.org/10.1016/S0169-7722(00)00109-1
Ciceri, G., Maran, S., Martinotti, W., & Queirazza, G. (1992). Geochemical cycling of heavy metals in a marine coastal area: Benthic flux determination from pore water profiles and in situ measurements using benthic chambers. Hydrobiologia, 235(236), 501–517.
Cleveland, D., Brumbaugh, W. G., & MacDonald, D. D. (2017). A comparison of four porewater sampling methods for metal mixtures and dissolved organic carbon and the implications for sediment toxicity evaluations. Environmental Toxicology and Chemistry, 36(11), 2906–2915. https://doi.org/10.1002/etc.3884
Crites, R. W., Watson, R. C., & Williams, C. R. (1995). Removal of metals in constructed wetlands Proceedings of WEFTEC 95, Miami, FL.
Cumming, G., & Finch, S. (2005). Inference by eye: Confidence intervals and how to read pictures of data. American Psychologist, 60, 170–180. https://doi.org/10.1037/0003-066X.60.2.170
Eger, P. (1994). Wetland treatment for trace metal removal from mine drainage: The importance of aerobic and anaerobic processes. Wat Sci Tech, 29(4), 249–256.
Harris, S., Xu, X., & Mills, G. L. (2020). Metal-sulfide dynamics in a constructed wetland in the Southeastern United States. Wetlands Ecology and Management, 28(6), 847–861. https://doi.org/10.1007/s11273-020-09749-6
Hesslein, R. H. (1976). An in situ sampler for close interval pore water studies. Limnology and Oceanography, 21, 912–914.
Kadlec, R. H., & Wallace, S. D. (2009). Treatment wetlands (2nd ed.). CRC Press.
Kerich, E. C. (2020). Households drinking water sources and treatment methods options in a regional irrigation scheme. Journal human Earth Future, 1, 10–19. https://doi.org/10.28991/HEF-2020-01-01-02
Kerndorf, H., & Schnitzer, M. (1980). Sorption of metals on humic acid. Geochimica Et Cosmochimica Acta, 44, 1701–1708.
Knox, A. S., Dunn, D. L., Paller, M. H., Nelson, E. A., Specht, W. L., & Seaman, J. C. (2006). Assessment of contaminant retention in constructed wetland sediments. Engineering in Life Sciences, 6, 31–36. https://doi.org/10.1002/elsc.200620116
Leermakers, M., Gao, Y., Gabelle, C., Lojen, S., Ouddane, B., Wartel, M., & Baeyens, W. (2005). Determination of high resolution pore water profiles of trace metals in sediments of the Rupel River (Belgium) using DET (diffusive equilibrium in thin films) and DGT (diffusive gradients in thin films) techniques. Water Air Soil Poll, 166(1–4), 265–286. https://doi.org/10.1007/s11270-005-6671-7
Machemer, S. D., & Wildeman, T. R. (1992). Adsorption compared with sulfide precipitation as metal removal processes from acid-mine drainage in a constructed wetland. Journal of Contaminant Hydrology, 9(1–2), 115–131. https://doi.org/10.1016/0169-7722(92)90054-I
Marchand, L., Mench, M., Jacob, D. L., & Otte, M. L. (2011). Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review (vol 158, pg 3447, 2010). Environmental Pollution, 159(2), 663–663. https://doi.org/10.1016/j.envpol.2010.11.010
Michaud, E., Desrosiers, G., Mermillod-Blondin, F., Sundby, B., & Stora, G. (2006). The functional group approach to bioturbation: II. The effects of the Macoma balthica community on fluxes of nutrients and dissolved organic carbon across the sediment-water interface. Journal of Experimental Marine Biology and Ecology, 337(2), 178–189. https://doi.org/10.1016/j.jembe.2006.06.025
Mills, G. L., Burgess, E. A., Murphy, T., Harmon, M., Mcleod, H., & Nelson, E. (2011). Biogeochemical processes in a treatment wetland mitigation metal discharge into a Savannah River Stream Tributary. https://smartech.gatech.edu/bitstream/handle/1853/46106/4.5.2Mills.pdf?sequence=1&isAllowed=y
Morse, J. W., & Richard, D. (2004). Chemical dynamics of sedimentary acid volatile sulfide. Environmental Science and Technology, 38, 131A-136A. https://doi.org/10.1007/s00244-010-9575-5
Nafchi, R. F., Samadi-Boroujeni, H., Vanani, H. R., Ostad-Ali-Askari, K., & Brojeni, M. K. (2021). Laboratory investigation on erosion threshold shear stress of cohesive sediment in Karkheh Dam. Environment and Earth Science, 80, 681. https://doi.org/10.1007/s00267-021-01567-7.
Nafchi, R. F., Yaghoobi, P., Reaisi Vanani, H., Ostad-Ali-Askari, K., Nouri, J., & Maghsoudlou, B. (2021b). Eco-hydrologic stability zonation of dams and power plants using the combined models of SMCE and CEQUALW2. Applied Water Science, 11(7), 109.
Ostad-Ali-Askari, K., Shayannejad, M., & Ghorbanizadeh-Kharazi, H. (2021). Artificial neural network for modeling nitrate pollution of groundwater in marginal area of Zayandeh-Rood River, Isfahan, Iran. KSCE Journal of Civil Engineering, 21, 34–140.
Pesch, C. E., Hansen, D. J., Boothman, W. S., Berry, W. J., & Mahony, J. D. (1995). The role of acid‐volatile sulfide and interstitial water metal concentrations in determining bioavailability of cadmium and nickel from contaminated sediments to the marine polychaete Neanthes arenaceodentata. Environmental Toxicology and Chemistry: An International Journal, 14(1), 129–141. https://doi.org/10.1002/etc.5620140115
Philipps, R., Xu, X., Bringolf, R., & Mills, G. (2018a). Impact of natural organic matter and increased water hardness on DGT prediction of copper bioaccumulation by yellow lampmussel (Lampsilis cariosa) and fathead minnow (Pimephales promelas). Environmental Pollution, 241, 451–458.
Philipps, R., Xu, X., Mills, G., & Bringolf, R. (2018b). Evaluation of diffusive gradients in thin-films for prediction of copper bioaccumulation by yellow lampmussel (Lampsilis cariosa) and fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 9999, 1–10. https://doi.org/10.1002/etc.4108
Philipps, R., Xu, X., Mills, G., & Bringolf, R. (2019). Evaluation of DGT technique for predicting uptake of metal mixtures by by yellow lampmussel (Lampsilis cariosa) and fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 38(1), 61–70.
Qin, C. Y., Xu, X. Y., & Peck, E. (2021). Metal removal by a free surface constructed wetland and prediction of metal bioavailability and toxicity with diffusive gradients in thin films (DGT) and biotic ligand model (BLM). Environmental Management. https://doi.org/10.1007/s00267-021-01567-7
Reddy, K. R., & Delaune, R. D. (2008). Biogeochemistry of wetlands: Science and applications. CRC Press.
Serbst, J. R., Burgess, R. M., Kuhn, A., Edwards, P. A., Cantwell, M. G., Pelletier, M. C., & Berry, W. J. (2003). Precision of dialysis (peeper) sampling of cadmium in marine sediment interstitial water. Archives of Environmental Contamination and Toxicology, 45, 297–230. https://doi.org/10.1007/s00244-003-0114-5
Sterne, J. A., & Smith, G. D. (2001). Sifting the evidence—What’s wrong with significance tests? British Medical Journal, 322, 226–230. https://doi.org/10.1136/bmj.322.7280.226
Truong, D. D., Tri, D. Q., & Don, N. C. (2021). The impact of waves and tidal currents on the sediment transport at the sea port. Civil Engineering Journal-Tehran, 7(10), 1634–1649. https://doi.org/10.28991/cej-2021-03091749
Xu, X., & Mills, G. L. (2018). Do constructed wetlands remove metals or increase metal bioavailability? Journal of Environmental Management, 218, 245–255. https://doi.org/10.1016/j.jenvman.2018.04.014
Xu, X., Mills, G. L., Lindell, A., Peck, E., Korotasz, A., & Burgess, E. (2019). The performance of a free surface and metal-removing constructed wetland: How a young wetland becomes mature. Ecological Engineering, 133, 32–38. https://doi.org/10.1016/j.ecoleng.2019.04.020
Xu, X., Peck, E., Fletcher, D. E., Korotasz, A., & Perry, J. (2020). Limitations of applying diffusive gradients in thin films to predict bioavailability of metal mixtures in aquatic systems with unstable water chemistries. Environ Toxi Chem, 1–11.
Zhang, H. (2003). DGT for measurements in waters, soils, and sediments. DGT Research LTD. http://fliphtml5.com/gwcd/wjjg/basic/51-58
Acknowledgements
We would like to thank Erin Peck, Cher Nicholson, John Perry, and Annah Nieman for their help on the field sampling, sample preparation, and data management and Angela Lindell for her assistance in the analytical work. We also want to express our appreciation to Dr. Gary Mills who established this project and served as the primary investigator from 2007 to 2017. This material is based upon work supported by the Department of Energy under Award Number DE-EM0004391 to the University of Georgia Research Foundation.
Author information
Authors and Affiliations
Contributions
XX: investigation, methodology, conceptualization, supervision, writing — original draft, review, and editing; ZEB: investigation, methodology, conceptualization, writing — review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, X., Baddar, Z.E. Metal fluxes at the sediment–water interface in a free water surface constructed wetland. Environ Monit Assess 194, 571 (2022). https://doi.org/10.1007/s10661-022-10258-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10258-7